An international collaboration led by Kobe University researchers has successfully developed a nanosheet-laminated photocatalytic (*1) membrane that demonstrates both excellent water permeance and photocatalytic activity. The membrane’s photocatalytic properties make it easier to clean as irradiating the membrane with light successfully reduces fouling (*2). They developed this membrane by laminating 2D nanomaterials (nanosheets (*3)) onto a porous support.
This revolutionary membrane technology can be applied to water purification, and thus has the potential to contribute towards tackling both global environmental and energy issues by helping to ensure safe drinking water supplies and clean energy. It is hoped that this will accelerate the move towards carbon neutral, sustainable societies.
This development was made by a research group at Kobe University’s Graduate School of Science, Technology and Innovation/Research Center for Membrane and Film Technology (Associate Professor NAKAGAWA Keizo, Professor YOSHIOKA Tomohisa and Professor MATSUYAMA Hideto) in collaboration with Professor TACHIKAWA Takashi of Kobe University Molecular Photoscience Research Center, Associate Professor Chechia Hu of National Taiwan University of Science & Technology and Professor Shik Chi Edman Tsang of Oxford University.
The results were first published in the ‘Chemical Engineering Journal’ on April 7, 2022.
Main points
- The researchers successfully developed a novel nanosheet-laminated photocatalytic membrane that demonstrates both excellent permeance and photocatalytic activity.
- The synergy of the nanosheet materials greatly enhanced both water permeability and photocatalytic activity.
- The photocatalytic property reduces membrane clogging (fouling).
- The researchers propose the application of this membrane to a water purification process using clean energy (light).
Research Background
Adequate access to water in many regions of the world is becoming an increasing problem in the face of global climate change, and developing countries’ sharp population increases and economic growth. It has been reported that two thirds of the world’s population will suffer water shortages by 2025. To prevent these severe water shortages, the widespread adoption of water recycling and purification technologies, as well as efficient usage of water production technologies (e.g. seawater desalination), are crucial.
The membrane filtration method is currently used in 900 water purification plants because it continuously and stably provides good quality water. However, there is the problem of membrane fouling where the membrane, which separates and removes contaminants from the water, becomes clogged. When membrane fouling occurs, it is no longer possible to obtain the required amount of treated water. Therefore, it is necessary to either wash or replace the membrane. To tackle this issue, much research has been conducted into various methods of fouling prevention however a sufficient solution has yet to be found.
One method has been proposed that requires less energy and has a low environmental impact. This involves introducing a photocatalytic material (such as titania) into the membrane and removing pollutants via photocatalysis. However, in addition to being able to treat water, such a membrane must also demonstrate visible light responsiveness and high photocatalytic activity. This requires the designer to consider the membrane’s design from multiple perspectives including the membrane material and structure.
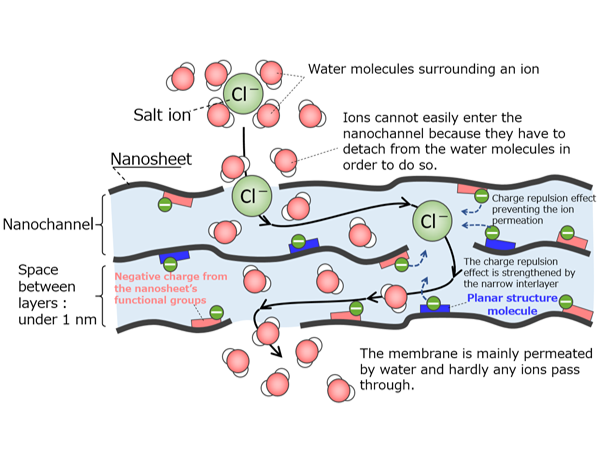
This research group previously developed a nanofiltration membrane, which works by utilizing 2D channels between its layers of nanosheets. They developed this membrane by laminating niobate nanosheets (*4) (a type of metallic oxide nanosheet, with each sheet being around a nanometer thick and a couple of hundred nanometers wide) onto a porous support membrane, which created the 2D channels between the nanosheets.
In this study, they discovered that adding carbon nitride nanosheets (which has visible light responsiveness) to the niobate nanosheet-layered membrane gave the membrane enhanced water permeance while greatly increasing photocatalytic activity. Furthermore, the photocatalytic properties of the membrane completely rectified the issue of the membrane’s permeance being reduced due to fouling.
Research Methodology
Nanosheet-laminated membranes can be formed by simple vacuum filtration of nanosheet materials (colloidal solutions) onto polymer support membranes. In this study, the research group produced an ultra-thin nanosheet-laminated membrane some 100 nanometers in thickness (Figure 1a). X-ray diffraction and molecular weight fractionation measurements revealed that introducing carbon nitride (*5) nanosheets into a niobate nanosheet-laminated membrane could control the diameter of nanochannels between the layers.
In terms of membrane functionality, the laminated nanofiltration membrane with a 74:25 ratio of niobate (HNB3O8) nanosheet to carbon nitride (g-C3N4) nanosheet maintained its separation performance while demonstrating an 8-fold increase in water permeance (Figure 1b). In terms of photocatalytic performance, the integration of carbon nitride nanosheets enabled visible light to be absorbed. In addition, this combination of nanosheets greatly improved the membrane’s ability to photodegrade cationic dyes (rhodamine B) (Figure 1c).

(a) Electron microscope image showing a cross-section of the nanosheet-laminated photocatalytic membrane developed in this study.
(b) Comparison of how different combinations of nanosheets affect water permeation speed.
(c) Changes in the rate constant of the rhodamine B photodegradation reaction depending on the combination of nanosheets (inset: photos showing the dye solution before and after photoirradition).
When the developed composite membrane is used as a separation membrane, the niobate nanosheets give the laminated membrane its structure, while the carbon nitride is introduced between these layers and acts as a spacer. Consequently, the channels in the laminated membrane expand, greatly increasing the rate of water permeation (Left side of Figure 2a). Controlling the channel structure in this way enables 90% of a dye (with molecular weight of approximately 1000) to be separated from the water. The photocatalytic functionality of the membrane is as follows: the carbon nitride nanosheets function as photocatalysts that absorb visible light and the niobate nanosheets act as catalytic promoters. Furthermore, the research group revealed that appropriately controlling the band structure enabled the electrons to move efficiently, resulting in a dramatic increase in photocatalytic activity (Right side of Figure 2a). Using these results as a basis, the researchers applied the membrane to water purification and conducted a membrane fouling experiment using Bovine Serum Albumin (BSA) as the foulant. BSA fouling reduced the water permeation speed of the membrane to 1/5 of its normal performance. However, the researchers succeeded in completely restoring its permeance by irradiating the composite nanosheet membrane (Figure 2b).

(a) The design of the novel nanosheet-laminated photocatalytic membrane, which uses two types of nanosheet, each with different functions.
(b) Changes in the relative water permeation speed of the photocatalytic membrane before and after photoirradiation. Bovine serum albumin (BSA) was used as the foulant. The performance of two different membranes was compared: a niobate (HNb3O8) nanosheet-laminated membrane and a composite niobate nanosheet/carbon nitride(HNb3O8/g-C3N4) nanosheet-laminated membrane.
Further Research
By interweaving different types of nanosheets to form 2D nanochannels, the researchers successfully developed a membrane that demonstrates both excellent water permeance and photocatalytic activity. It is expected that further improvements can be made to membrane functionality and photocatalytic action by changing the type of nanosheet to more precisely control the formation of 2D nanochannels and the band structure. Next the researchers hope to increase the membrane area and develop the photocatalytic process, aiming for industrial and practical application.
Glossary
1. Photocatalyst
A material that demonstrates catalysis (speeds up a reaction) when illuminated by light. Excited electrons and holes generated inside the photocatalyst when it absorbs light result in reduction and oxidation, respectively. This study utilizes a photocatalyst for the oxidative degradation of an organic compound.
2. Fouling
The phenomenon whereby the substances to be separated (foulants) from the water adhere to and accumulate on the membrane’s surface and pores.
3. Nanosheet
A colloidal material with a molecular thickness and a planar size of several hundred nanometers to micron order. In other words, an extremely thin 2D structure that is invisible to the naked eye. Studies have reported on various types of nanosheets such as metallic oxide nanosheets (e.g. graphene oxide).
4. Niobate nanosheet
A type of metallic oxide nanosheet. Nanosheet crystals are formed by horizontally connecting the octahedral structure of NbO6-. It is used as a photocatalyst and as a solid acid catalyst.
5. Carbon Nitride
A family of materials. In this study, a planar structure consisting of hydrogen, carbon and nitrogen atoms (graphitic carbon nitride, g-C3N4). It is a semiconductor with a small bandgap, and is a visible light-responsive photocatalyst.
Acknowledgements
This study was supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (c) (Grant Number: JP19K05121) and the Ministry of Science and Technology, Taiwan (Grant Number: 110-2221-E-011-012-MY3).
Journal Information
Title
DOI
10.1016/j.cej.2022.136254
Authors
Seiji Imoto, Keizo Nakagawa, Chechia Hu, Tomohisa Yoshioka, Takuji Shintani, Atsushi Matsuoka, Eiji Kamio, Takashi Tachikawa, Shik Chi Edman Tsang, Hideto Matsuyama
Journal
Chemical Engineering Journal