The first analysis of how synaptic proteins change during early development reveals differences between mice and marmosets but also what's different in individuals with autism spectrum disorders. The Kobe University findings offer first insights into the mechanism behind synaptic development and open up routes for research on possible treatments.
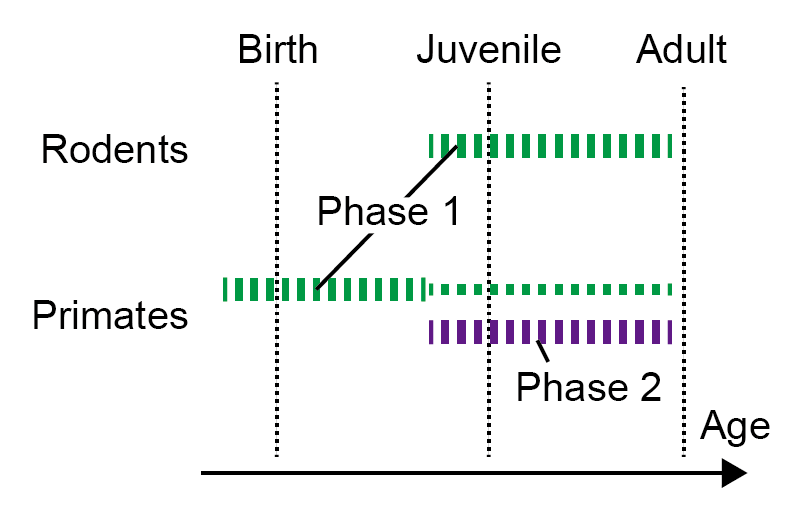
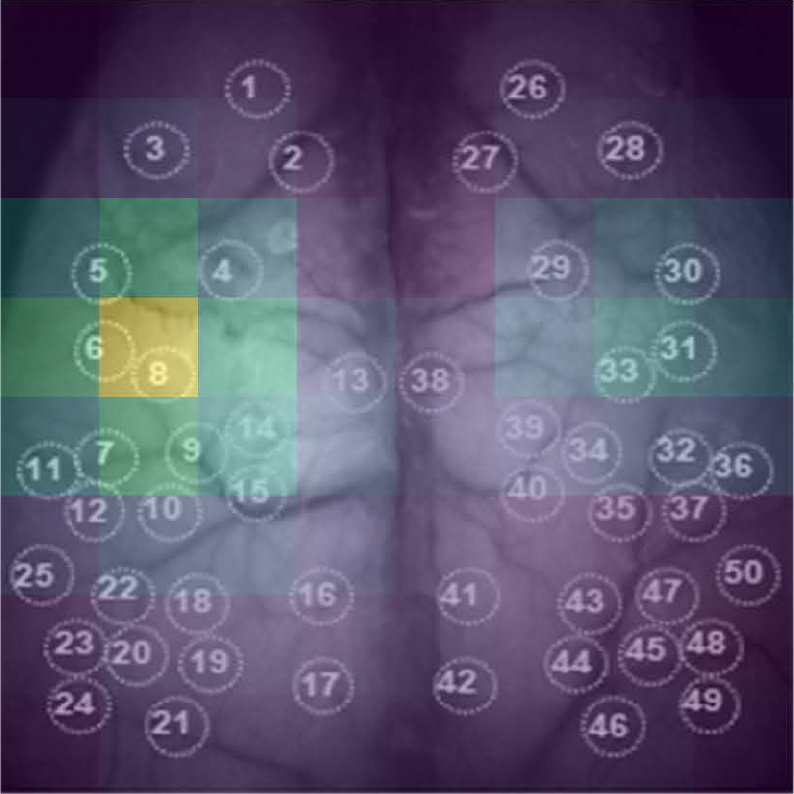
The Kobe University-led research team identified a group of proteins that undergo a phase of change where some are produced more and others are produced less as time passes. They also found that the timing of this change is different in mice and marmosets: What happens in mice two weeks after birth happens before or around birth in marmosets. In addition, marmosets have a second phase of protein changes that mice don’t have. © KAIZUKA Takeshi (CC BY)
Given that synapses are the connections between our brain cells, one might think that having as many of these as possible is a good thing. However, primate brains do something unexpected: After early childhood, the connections between brain cells start to decrease in a process called “synaptic pruning.” Surprisingly, we know very little about the molecular mechanism behind how synapses change as brains mature and this is also a hurdle for the development of cures to neuropsychological disorders such as the autism spectrum disorder.
Both recent developments in the ability to analyze complex protein assemblies and the recent availability of marmosets as non-human primate model organisms for studies on the brain enabled Kobe University neuroscientist TAKUMI Toru to tackle this knowledge gap. He explains, “The Collaboration with experts in proteomics and non-human primate brain has been a critical factor for enabling this study. Also, we have established an analytical pipeline to compare multiple biological data sets using the latest statistical and bioinformatics tools, which was another crucial element.” With this, they focused their studies on the analysis of a protein agglomeration found on the signal-receiving side of synapses, the so-called “postsynaptic density,” as it has become clear in previous studies that its constituents are key to the development of small mushroom-shaped protrusions on the signal-receiving cells where synapses are formed.
Their results, published in the journal Nature Communications, are science’s first look at what is happening at the protein level in synapses during brain development in the first weeks, months and years after birth. The Kobe University-led research team identified a group of proteins that are produced more and others that are produced less as time passes and could confirm that this is due to changes in gene regulation. They also found that the timing of this regulation is different in mice and marmosets: What happens in mice two weeks after birth happens before or around birth in marmosets. In addition, marmosets have a second phase of protein changes that mice don’t have. “This may be related to the evolutionary differences between rodent and primate brains,” comments KAIZUKA Takeshi, the first author of the paper, also in respect to the process of synaptic pruning.
Takumi’s interest didn’t stop there. Knowing that the development of autism spectrum disorder is connected to developmental immaturities of synapses, they investigated what this means on the level of the proteins his group had identified to be connected to synapse development. And indeed, they found that the genes reported to be expressed differentially in autism patients also feature prominently in their data. “These data suggest that the postsynaptic density in autism spectrum disorder patients is relatively similar to that in the prenatal or neonatal period compared to healthy subjects,” the researchers write in the study. Being thus able to construct hypotheses on the molecular mechanism behind the emergence of the disorder, this might open up the path for the development of treatments.
Takumi sums up the implications of this study. “Synapse development is a crucial issue to consider in the maturation of brains. Its abnormalities are related to neuropsychiatric disorders, including autism spectrum disorders and schizophrenia. The proteome datasets we provided are important for considering molecular mechanisms of synapse development and the difference between rodents and primates.”
Acknowledgements
This research was funded by the Japan Society for the Promotion of Science (grants 16H06316, 16H06463, 18K14830, 21H04813, 23H04233, 23KK0132 and 16J04376), the Japan Agency for Medical Research and Development (grants JP21wm0425011 and JP20dm0207001), the Japan Science and Technology Agency (grants JPMJMS2299 and JPMJMS229B), the National Center of Neurology and Psychiatry (grant 30-9), the Takeda Science Foundation, and the Taiju Life Social Welfare Foundation. It was conducted in collaboration with researchers from the RIKEN Center for Brain Science, the RIKEN Center for Sustainable Resource Science, the Max Planck Institute for Experimental Medicine, Hokkaido University and Keio University.
Original publication
T. Kaizuka et al.: Remodeling of the postsynaptic proteome in male mice and marmosets during synapse development. Nature Communications (2024). DOI: 10.1038/s41467-024-46529-9
Release on EurekAlert!
Synaptic protein change during development offers clues on evolution and disease