Kobe University Biosignal Research Center has discovered the first molecular evidence that cell motility, a key component in immunity, development, and other fundamental biological mechanisms, is regulated by a physical factor—membrane tension stress. This finding is expected to have significant implications for treating infections and malignant tumors, and was published on-line in a top peer-reviewed journal, Nature Cell Biology , on May 4, 2015, London time.
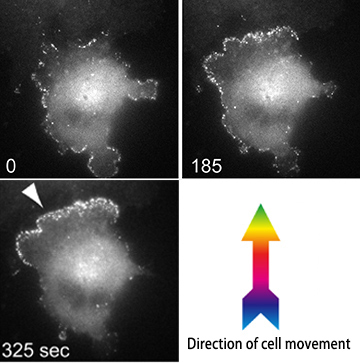
FBP17–actin complexes (white dots) distributed across the cell membrane are recruited over time to the leading edge of migration due to increased tension.
Led by Prof. ITOH Toshiki, Assistant Prof. TSUJITA Kazuya and other researchers in Prof. ITOH’s laboratory conducted studies using a cultured tumor cell line, and showed that tension stress on the plasma membrane plays a major role in the regulation of cell motility. More specifically, this group showed that formin-binding protein-17 (FBP17), a protein known to bend the plasma membrane, acts as a tension sensor that mediates the direction of cell migration.
In the human body, cell motility is regulated to support immune homeostasis and growth. For example, when bacteria or viruses enter the body, macrophages, neutrophils, and other immune cells identify, trace, and eliminate them. During development, when a fertilized egg undergoes proliferation and differentiation, new cells migrate to the appropriate sites via strictly controlled mechanisms. Disruption of the mechanisms regulating cell motility may be a key factor in cancer cell metastasis. Thus, elucidation of the mechanisms governing cell movement will be a critical step toward overcoming malignant tumors.
Prof. ITOH says, “We are promoting research from a less studied perspective to identify new approaches to cancer treatment.”
Journal information
- Title
- “Feedback regulation between plasma membrane tension and membrane-bending proteins organizes cell polarity during leading edge formation”
- DOI
- 10.1038/ncb3162
- Authros
- Kazuya Tsujita, Tadaomi Takenawa, Toshiki Itoh
- Journal
- Nature Cell Biology