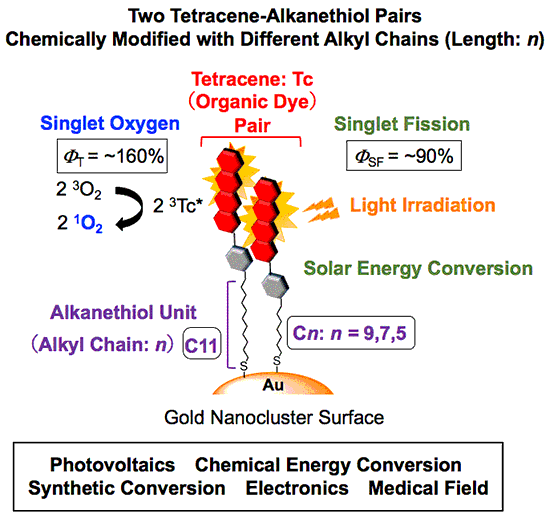
A research group comprising Associate Professor Taku Hasobe and Assistant Professor Hayato Sakai of Faculty of Science and Technology in the Keio University, Toshiyuki Saegusa of Graduate School of Science and Technology in the Keio University (completed master's program in 2019), and Professor Yasuhiro Kobori and postdoctoral researcher Hiroki Nagashima of Molecular Photoscience Research Center in the Kobe University found that when light was exposed to the surface of a tetracene alkanethiol-modified gold nanocluster, which they developed themselves, twice as many excitons could be converted compared to the number of photons absorbed by the tetracene molecules. They also found that these excitons have a lifetime that is approximately 10,000 times longer than that of the organic molecules on conventional gold surfaces. Furthermore, they succeeded in converting singlet oxygen (a type of reactive oxygen species) at a highly efficient conversion rate of 160%, far exceeding 100% conversion, in comparison to the number of absorbed photons. Singlet oxygen is used in photodynamic therapy (treatment of cancer with light) and organic synthesis, among other applications.
These findings are expected to contribute to areas such as solar energy conversion, electronics, life sciences, and medical care in the future. The outcomes of this research were published in the online version of the American scientific publication the "Journal of the American Chemical Society" on September 6.
Main points of research
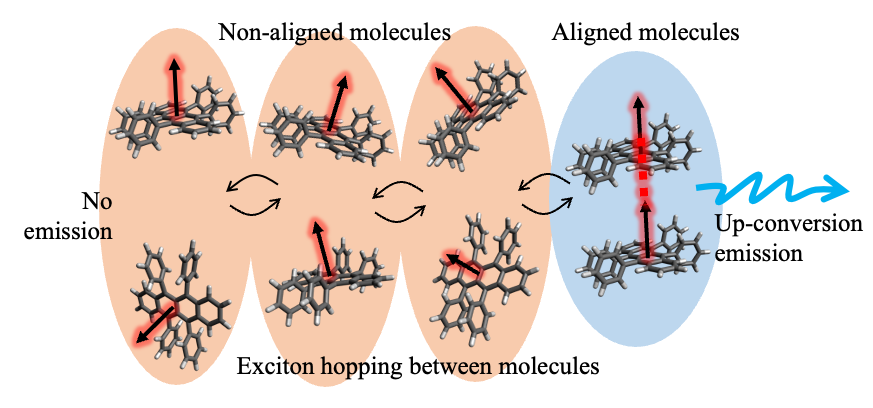
- Normally, when one photon is absorbed by a molecule, only one exciton (a bound state of an electron hole and an electron) is formed. However in recent years, singlet fission (which forms two excitons from the absorption process of a single photon) is gathering much attention worldwide, although significant work remains before it can be used in chemical reactions.
- In general, an organic molecule that has been chemically modified and integrated into the surface of metals loses significant excitation energy when compared to the isolated state of an organic molecule.
- In order to solve all of the above problems at once, a tetracene alkanethiol-modified gold nanocluster was newly designed and synthesized. An increase in lifetime of about 10,000 times was achieved by greatly suppressing the rapid loss of excitation energy on the metal surface. In addition, excitons were formed with high efficiency through singlet fission, and the efficiency of generating singlet oxygen was successfully improved to about 160% (fig. 1).
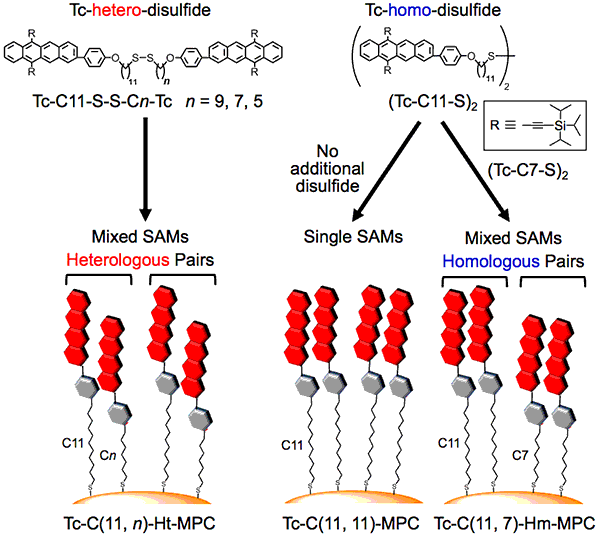
Content of research and results
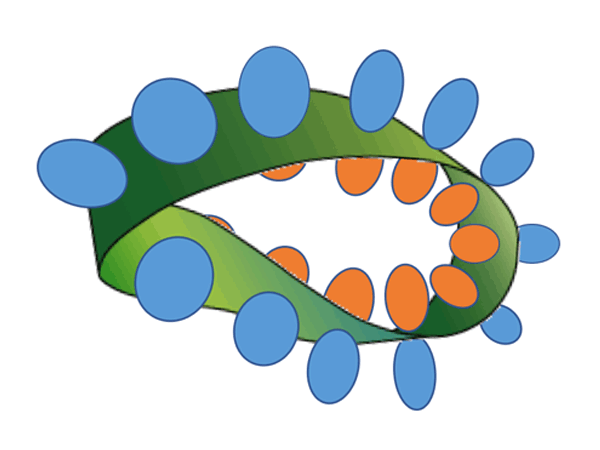
A research group comprising researchers from Keio University, Kobe University, and Tampere University focused on a photoreaction called singlet fission. This is a process in which two molecules positioned nearby interact with each other after one of the molecules absorbs a photon, forming two excitons. With the goal of solving the abovementioned problems all at once, they considered modifying tetracene (whose chemical structure is composed of four benzene rings connected in a straight line) into a metal nanocluster by the self-assembled monolayers (SAMs) method (fig.2). SAMs are monolayers made by chemically modifying organic ligands such as alkanethiol on the metal surface, and have been an important core technology for recent advancements in nanotechnology. In singlet fission, where reactions take place between two molecules located close to each other, the distance and orientation between the two molecules must be strictly controlled. When the surface of a gold nanocluster is chemically modified using tetracene homodisulfide (fig.2, right) that is composed of two tetracene alkanethiol with the same alkyl chains of length n, the probability of tetracene with the same alkyl chains of length n being placed in close proximity inevitably increases. Therefore, as shown on the left side of figure 2, tetracene heterodisulfide (Tc-C11-S-S-Cn-Tc) with different alkyl chain lengths (for which one had a length of n = 11 while the other had a length of n = 5, 7, or 9), were newly synthesized, and the gold nanocluster surface was chemically modified. Using tetracene heterodisulfide and tetracene homodisulfide, a series of tetracene alkanethiol-modified gold nanoclusters was synthesized (a block [cluster] of gold with 144 gold atoms whose surface was chemically modified by 60 tetracenes with alkanethiol as the medium). As a result, it was possible to create bimolecule arrangements with the optimal distance and orientation for singlet fission to occur efficiently on the gold nanocluster surface (while suppressing reverse reactions).
Journal information
- Title
- “Controlled Orientations of Neighboring Tetracene Units by Mixed SAMs on Gold Nanoclusters for High-Yield and Long-Lived Triplet Excited States through Singlet Fission”
- DOI
- 10.1021/jacs.9b06567
- Authors
- Toshiyuki Saegusa, Hayato Sakai, Hiroki Nagashima, Yasuhiro Kobori, Nikolai V. Tkachenko, and Taku Hasobe
- Journal
- Journal of the American Chemical Society