A research group led by Associate Professor Takashi Tachikawa of Kobe University’s Molecular Photoscience Research Center has succeeded in developing photocatalysts that can convert an efficient level of hydrogen from water using solar light. It is hoped that methods like this one, which uses titanium-modified hematite mesocrystal-based photoanodes, could form the foundation for a commercial solar water splitting system. This would allow the clean fuel hydrogen to be produced more cheaply and easily than before, making it a viable source of renewable energy.
This was a joint research project with Nagoya University’s Institute of Materials and Systems for Sustainability (Professor Shunsuke Muto) and the Japan Synchrotron Radiation Research Institute (JASRI) (Chief Researchers Koji Ohara and Kunihisa Sugimoto).
The results of this study were first published in the online journal Nature Communications on October 23 2019.
Background
As environmental and energy problems increase, hydrogen has been receiving more attention as a possible clean energy source of the future. Photoelectrochemical (PEC) water splitting (also known as solar water splitting) has been proposed as renewable way to produce hydrogen. In theory, it is a simple method which requires a photocatalyst and sunlight to obtain hydrogen from water. Industrial-scale PEC water splitting systems would lower the commercial price of hydrogen, making it a practical energy source.
However, in order to make PEC water splitting a viable method of producing hydrogen on a large scale, the light-to-energy conversion efficiency needs to be improved. When the photocatalyst is exposed to light, electrons and holes (made by the electrons) are formed on the surface of the photocatalyst. These charges then dissociate to produce hydrogen and oxygen from water molecules. Although experiments with many different photocatalysts have been carried out, a reoccurring problem is that the electrons and holes recombine on the catalyst surface, lowering the conversion efficiency. Other issues include catalyst durability and cost.
In order to control the dynamics of electrons and holes via precise alignment of nanoparticles, Associate Professor Tachikawa et al developed a method using ‘hematite mesocrystal-based photoanodes’ as a photocatalyst. They succeeded in producing a highly efficient light to energy conversion. Mesocrystals are superstructures of nanoparticles with highly ordered structures. This makes them efficient for charge separation and transport. Furthermore, hematite is an abundant natural mineral- making this a potentially low cost method.
Research methodology
Mesocrystal-based photoanodes:
The mesocrystals with highly ordered nanoparticles were made via solvothermal synthesis (a method of chemical compound production using high pressure and temperature). These were then used to develop the mesocrystal-based photoanode. This high temperature exposure formed oxygen vacancies, Vo (small oxygen deficient spaces) inside the mesocrystals due to the partial fusion of the interface between the nanocrystals (Figure 1). This increased the carrier density of the mesocrystals, thus improving their conductivity further. Examinations of the composition and structure of the mesocrystals also revealed pores on the surface of the particles (see Figure 2 for more information). These mesopores and particle attachments appear to aid light absorption and charge mobility, respectively.
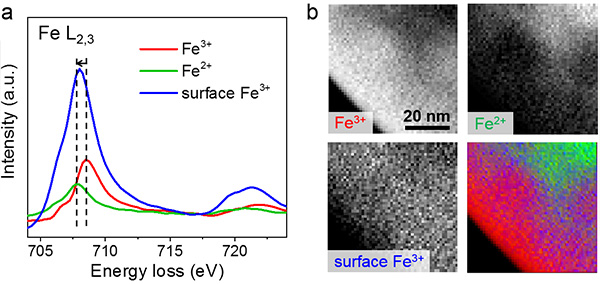
a. EEL (Electron Energy Loss) spectra of Fe L2,3 using multivariate analysis
b. Image of the spatial distribution of iron elements in and on the mesocrystals. From the high background intensity, the Fe2+ oxide is considered to be mostly located inside the mesocrystals. Vo are likely to form in the regions where neighboring nanoparticle fusion took place.
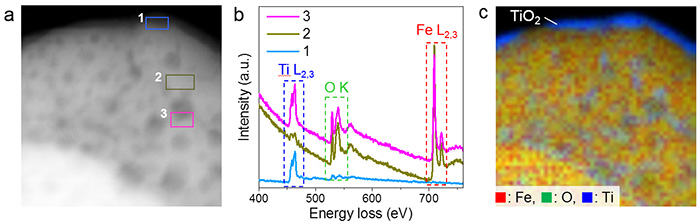
a. HAADF-STEM image of the mesocrystal after being exposed to 700 °C. The dark spots on top of the particle are pores.
b. EELS spectra of the selected regions in image a. show that for Region 1 (the outside surface of the mesocrystal)- titanium and oxygen are present, but there is hardly any iron. There is comparatively less titanium in Region 2 (the smooth surface of the mesocrystals) than in Region 3 (edges of the mesocrystal pores).
c. Chemical composition map of iron, oxygen and titanium
As previously mentioned, one of the main issues with PEC water splitting is that the electrons and holes recombine before the water splitting reaction (the separation of oxygen and hydrogen in water molecule) can efficiently take place. It was suggested that the electron-hole pairs generated near the Vo have longer lifetimes. This would make it easier for the holes to escape from recombination with the photogenerated electrons- improving the conversion performance.
Titanium-modified hematite:
The photoanodes were constructed using titanium-modified hematite (Ti-Fe2O3) mesocrystals. Ti modification was carried out with the aim of increasing the conductivity and ease of charge separation.
A solar water splitting method was set up as shown in detail in Figure 3. The Ti-modified hematite photoanodes were placed in an alkaline water solution under illumination with simulated sunlight. A platinum (Pt) electrode is used as the cathode. The oxygen molecules are generated from the mesocrystal-based photoanode and the hydrogen molecules are produced from the Pt counter electrode.
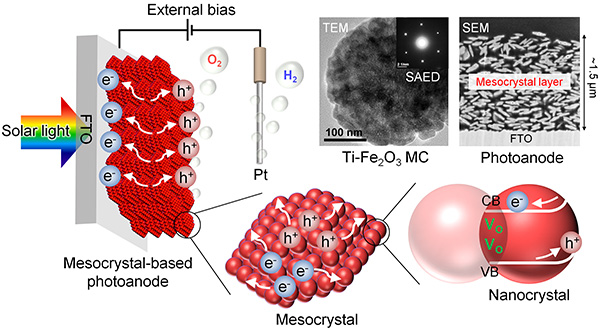
The above diagram shows oxygen vacancies (Vo), and the movement of electrons (e-) and holes (h+). Using TEM (Transmission Electron Microscopy), it is possible to see the arrangement of nanoparticles inside the mesocrystals. SAED (Selected Area Electron Diffraction) was also carried out in order to examine the structure of the mesocrystals in more detail- indicating that the nanocrystals inside are highly ordered and aligned. The SEM image of the mesocrystal layer shows the disc-shaped mesocrystals and the network of pores and particles that aid light absorption and charge mobility, respectively.
Next, tests were carried out to determine the photocurrent density of the photoanodes. A photocurrent is a reverse current which results from the electrons and holes travelling towards the cathode and anode respectively. A high photocurrent density would indicate a strong conversion efficiency from sunlight to hydrogen through PEC water splitting.
The photocurrent densities of Ti-modified hematite photoanodes with different film thicknesses were compared under two illumination modes. It was found that back illumination (where the surface of the hematite is illuminated through the FTO glass) generated more current in all samples than front illumination (where the light has to pass through the electrolyte before reaching the hematite). The most efficient film thickness was shown to be 900 nm. These photoanodes were shown to have a photocurrent density of 2.5 mAcm-2 at a potential of 1.23v.
This method, using back illumination, also solves the problem of light scattering caused by the evolved gas bubbles. Light scattering is another issue that can reduce the conversion efficiency. It was also found that adding a Co-Pi (Cobalt phosphate ion) co-catalyst to the surface of the photoanodes further improved the photocurrent density to 3.5mAcm-2 (Figure 4). This photocurrent density is the highest achieved so far using hematite as the photocatalyst material under back illumination.
During solar water splitting, the evolved gases H2 and O2 were produced over a three hour time period at a stoichiometric ratio of 2:1 (Figure 4). Furthermore, the photoanodes did not exhibit any obvious decrease in current over a 24 hour period, suggesting stability under extended operating conditions.
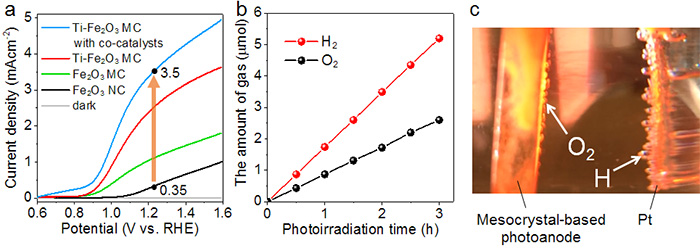
A. Graph comparing the current density for different types of photoanodes. The Ti-modified hematite mesocrystal-based photoanodes with Co-Pi co-catalysts exhibit the best performance. The potential is expressed versus the RHE (Reversible Hydrogen Electrode). The electrochemical water splitting occurs at a standard potential of. 1.23 V.
B. The amount of evolved gases produced from PEC water splitting over a 3 hour period. The ratio of hydrogen to oxygen is 2:1.
C. Photograph of the photoanode and Pt counter electrode.
Conclusions:
This research showed that Ti-modified hematite mesocrystal photoanodes demonstrate a high generation efficiency of hydrogen from water under back illumination. The analyses carried out during the course of this study suggest that these photoanodes with Vo and mesopores have properties that make them highly suited to solar water splitting- including efficient light absorption, long-lived holes and superior charge mobility. However, some recombination issues in the film still remain. Performance could be further improved through surface treatment.
Further academic and industry research collaborations into conversion rate improvement and the suitability of other kinds of mesocrystal photoanodes could lead to the prompt realization of a commercial PEC water splitting system.
Glossary
- FTO
- (Fluorine doped Tin Oxide) glass is a durable and electrically conductive thin glass film.
- STEM
- (Scanning Transmission Electron Microscopy): a type of TEM (Transmission Electron Microscope) that can focus on a microscopic area to create images
- EELS (electron energy loss spectroscopy):
- A material is exposed to a beam of electrons with a known, narrow range of kinetic energies. The reaction of these electrons is useful for detecting the elemental components of a material. In combination with STEM, this can be used to map a sample at atomic resolution.
- HAADF-STEM (High-angle Annular Dark-Field Scanning Transmission Microscopy)
- imaging method that allows heavy atoms to be brightly observed.
Journal information
- Title
- “Interfacial oxygen vacancies yielding long-lived holes in hematite mesocrystal-based photoanodes”
- DOI
- 10.1038/s41467-019-12581-z
- Authors
- Zhujun Zhang, Izuru Karimata, Hiroki Nagashima, Shunsuke Muto, Koji Ohara, Kunihisa Sugimoto, Takashi Tachikawa
- Journal
- Nature Communications