The mechanism behind oil synthesis within microalgae cells has been revealed by a Japanese research team. This discovery could contribute to the development of biofuels. The findings were published on April 4 in Scientific Reports.
The research was carried out by a group led by Professor HASUNUMA Tomohisa and Academic Researcher KATO Yuichi, both from the Kobe University Graduate School of Science, Technology and Innovation.
During the 20th century the petrochemical industry developed rapidly, leading to depletion of fossil resources and climate change on a global scale. In order to solve these issues and realize a sustainable and environmentally-conscious society, we must make use of renewable biomass such as plants and algae.
The amount of biomass on Earth is approximately 10 times the amount of energy we currently consume. Roughly half of this biomass grows in aquatic environments, and ocean-based biomass such as microalgae can produce oil without using up arable land and drinking water.
Microalgae can grow with light, water, carbon dioxide and a small amount of minerals, and their cells divide quickly, meaning that they can be harvested faster than land-based biomasses. Algae can also be harvested all year round, potentially offering a more stable energy supply.
Many species of algae are capable of producing large amounts of oil (lipids), but this is the first time that researchers have captured the metabolic changes occurring on a molecular level when lipids are produced in algae cells.
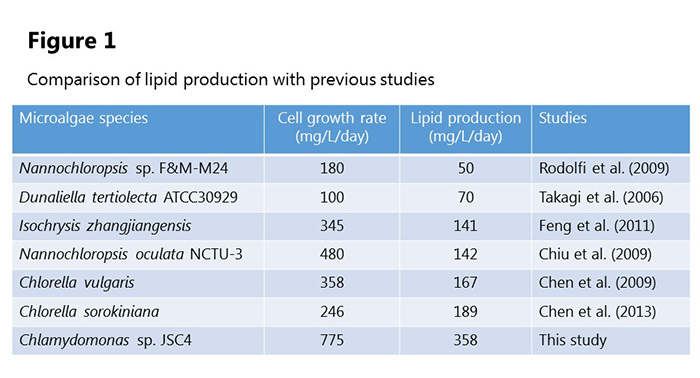
Focusing on marine microalgae, Professor Hasunuma’s group found that Chlamydomonas sp. JSC4, a new species of green alga harvested from brackish water, combines a high growth rate with high levels of lipids. The research team developed an analysis method called “dynamic metabolic profiling” and used this to analyze JSC4 and discover how this species produces oil within its cells.
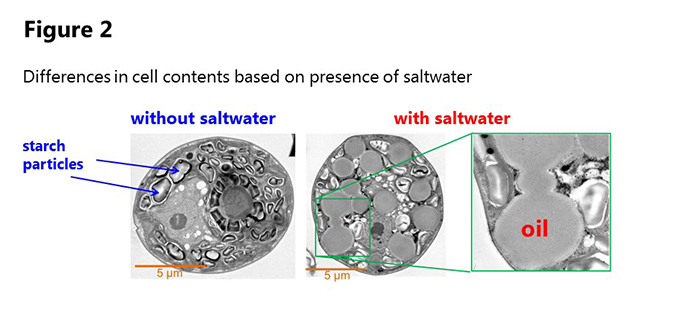
Professor Hasunuma’s team incubated JSC4 with carbon dioxide as the sole carbon source. 4 days after the start of incubation, over 55% of cell weight consisted of carbohydrates (mainly starch). When saltwater comprised 1-2% of the incubation liquid, the team saw a decrease in carbohydrates and increase in oil, and 7 days after the start of incubation over 45% of cell weight had become oil.
JSC4 has a high cell growth rate, and the lipid production rate in the culture solution achieved a speed that greatly surpassed previous experiments. At the start of the cultivation period starch particles were observed in the cells, but in saltwater these particles vanish and numerous oil droplets are seen (figure 1).
Using dynamic metabolic profiling, the group found that the sugar biosynthesis pathway (activated when starch is produced) slows down, and the pathway is activated for synthesizing triacylglycerol, a constituent element of oil. In other words, the addition of seawater switched the pathway from starch to oil production. They also clarified that the activation of an enzyme that breaks down starch is increased in saltwater solution.
The discovery of this metabolic mechanism is not only an important biological finding, it could also be used to increase the production of biofuel by improving methods of algae cultivation. Based on these findings, the team will continue looking for ways to increase sustainable oil production by developing more efficient cultivation methods and through genetic engineering.
Journal information
- Title
- Dynamic metabolic profiling together with transcription analysis reveals salinity-induced starch-to-lipid biosynthesis in alga Chlamydomonas sp. JSC4
- DOI
- 10.1038/srep45471
- Authors
- Shih-Hsin Ho, Akihito Nakanishi, Yuichi Kato, Hiroaki Yamasaki, Jo-Shu Chang, Naomi Misawa, Yuu Hirose, Jun Minagawa, Tomohisa Hasunuma*, Akihiko Kondo
- Journal
- Scientific Reports