Clarifying the cause of a skin disease led to the discovery of a new disease-causing gene, a new category of diseases, and new perspectives for both counseling and therapy. The Kobe University discovery is the first time that epigenetic silencing, the “switching off” of an otherwise intact gene, has been recognized as the cause for a skin disease.
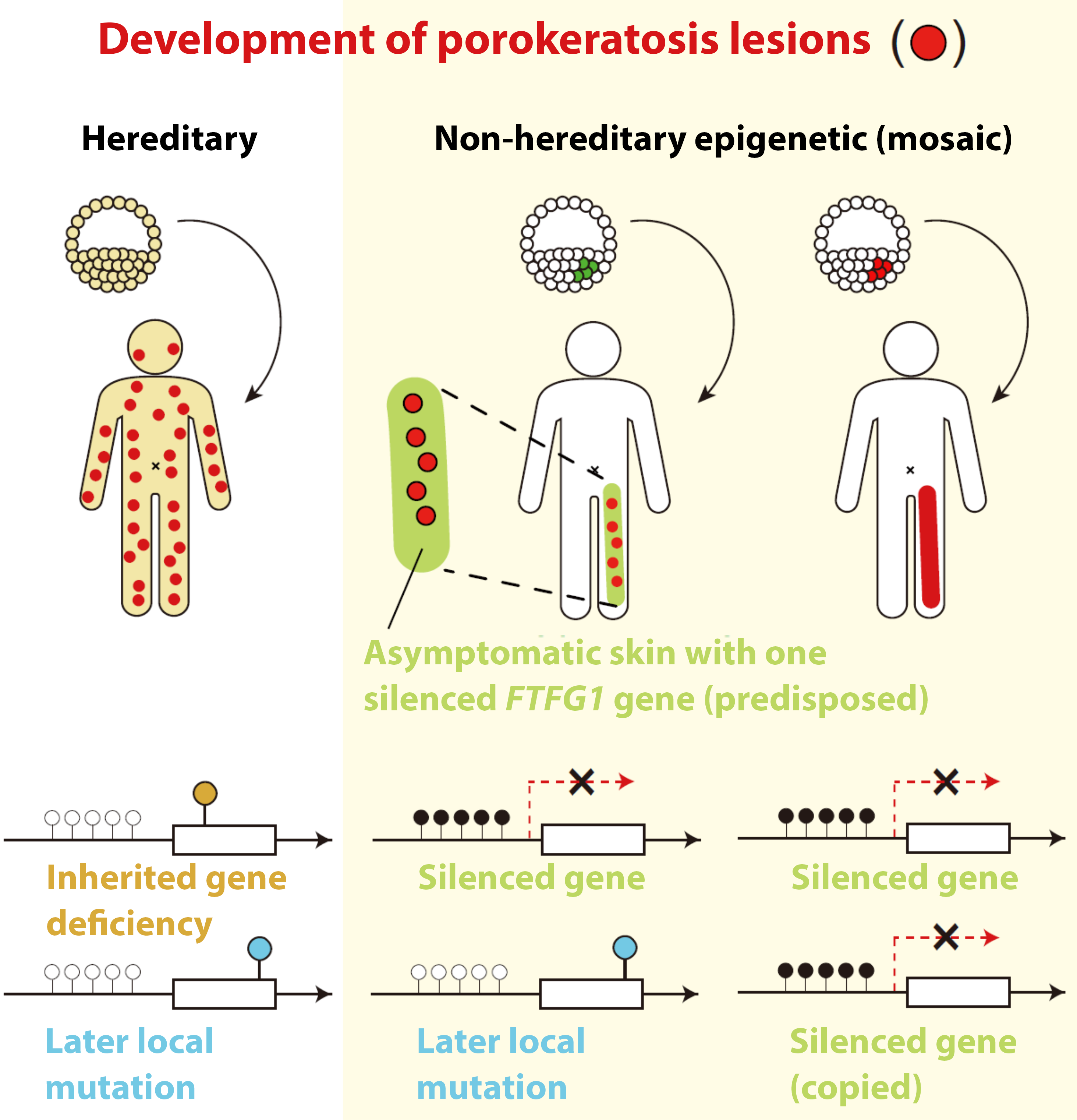
Porokeratosis is a skin disease that leads to the development of annular or circular, red and itchy lesions. In some individuals, these develop all over the body, in some localized in lines, and in some only in one or very few spots. Kobe University dermatologist KUBO Akiharu previously discovered that patients need “two hits” to one of the four genes that, when damaged, were known to cause the disease. Kubo explains: “We get one set of genes from each of our parents, which means that, for all genes relevant to this disease, we have two copies. However, patients had one deficient copy in all of their cells, which means that they inherited that from one of their parents. But they also had later mutations in the other copy in those areas of the skin where the disease developed.” After that discovery, over fifty patients visited Keio University Hospital and Kobe University Hospital to get screened by Kubo, and among these they found eight patients who didn’t have deficiencies in any of the four genes known to cause the disease, and they also had slightly different symptoms. “I was convinced that there is a yet unknown cause of porokeratosis, and so my graduate student at Keio University, SAITO Sonoko, started to search for it.”
In The American Journal of Human Genetics, they now report that they identified a new gene, called FDFT1, that when damaged will cause porokeratosis. But while those patients who had lesions all over the body had one deficient copy inherited from one of the parents and one later mutation in the affected cells, which is similar to what is known for other causative genes, those with more localized lesions did not have such an inherited damaged copy. Kubo says, “These observations led to the hypothesis that not genetic, but epigenetic changes in FDFT1 are hidden as the first hits.” Epigenetic changes don’t affect the DNA sequence that constitutes the gene but refer to molecular tags a cell can add to DNA to indicate whether or not to produce proteins from the gene, depending on the tag. “And that is exactly what we found. Epigenetic silencing of FDFT1 during early embryonic development in a cell that will give rise to skin cells is the first hit in this type of porokeratosis.”
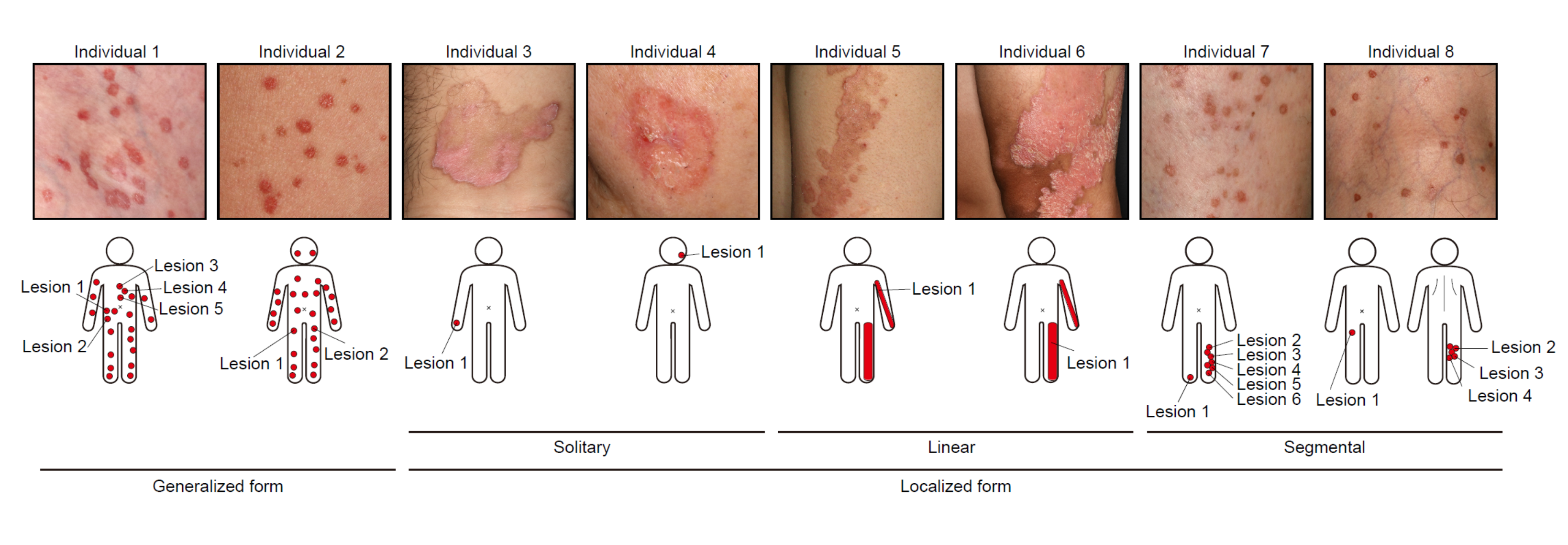
The whole picture therefore is that, in order to develop the disease, people need two damaged copies of the gene, “two hits.” One they acquire either from their parents or through epigenetic silencing early in their fetal development. This predisposes them to developing the disease but in itself is not symptomatic. In the case of epigenetic silencing, individuals are mosaics of predisposed cells, which derive from the one where the silencing occurred, and unaffected cells. A second defect in the other copy of the gene leads to the development of the disease: Similar to the previously known causative genes, the protein produced from FDFT1 is involved in the production of cholesterol and with both copies of the gene deficient, toxic by-products accumulate in the cells.
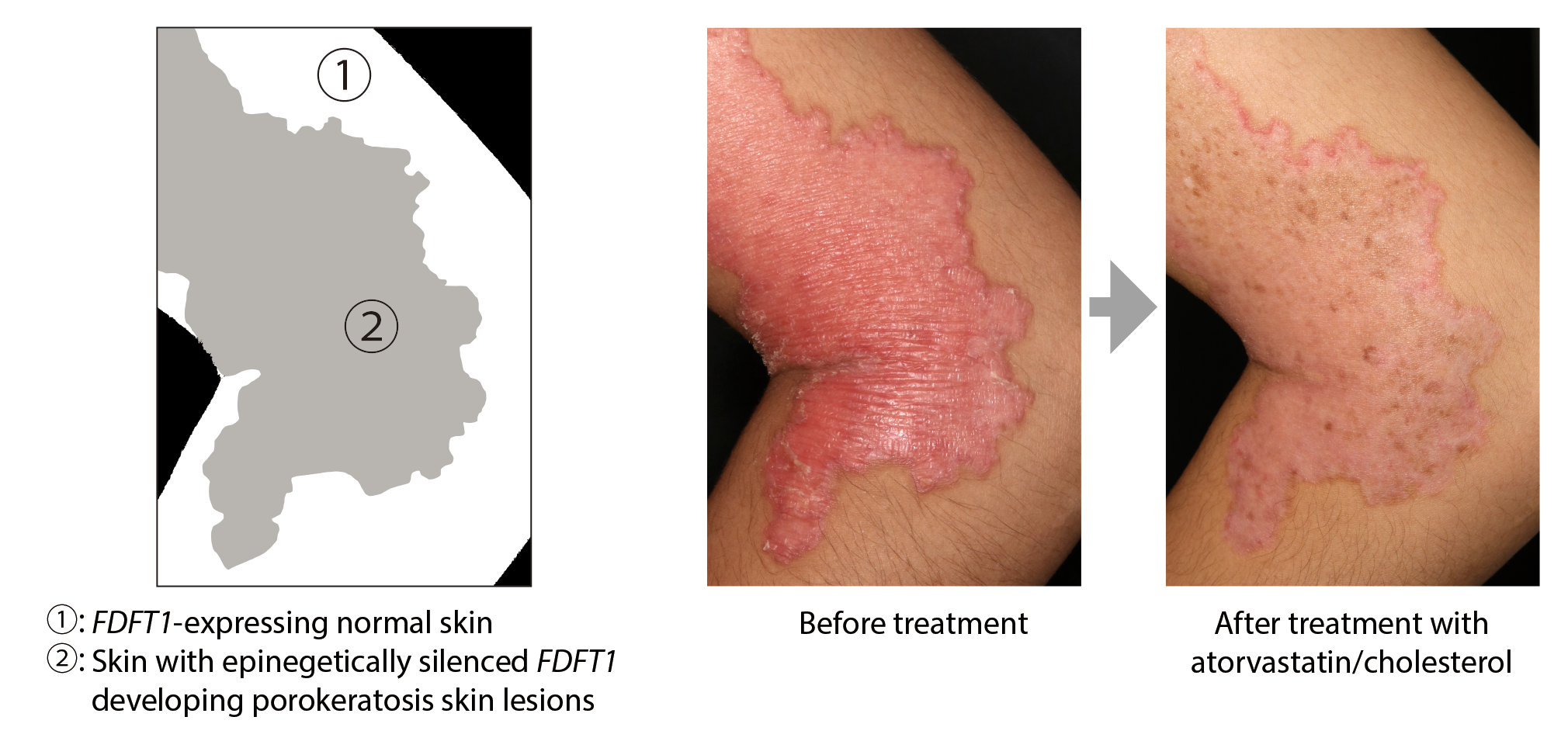
These results have many fascinating implications. First, the knowledge of the affected gene allows doctors to prescribe a treatment. “We conducted a therapeutic evaluation of atorvastatin (a blocker of cholesterol production) and cholesterol ointment in three individuals with FDFT1-deficient porokeratosis. All individuals exhibited reduced skin redness and thickening, pruritus (itchiness), and scaling within 4-12 weeks of treatment initiation, and no relapse was observed with continued use of the ointment,” the researchers write in the paper. Second, when counseling patients, for those who didn’t inherit a deficient gene from their parents it is reassuring news that they will also not pass the predisposition to the illness on to their children.
And third, “This is the first skin disease caused by early-development epigenetic silencing of a particular gene. Among all diseases, a fully comparable mechanism is only known in Lynch syndrome. We thus expect that epigenetic causes are hidden not only in these but also in other diseases, suggesting the existence of a category of diseases associated with the silencing of genes,” the researchers explain.
Acknowledgements
This research was funded by the Japan Society for the Promotion of Science (grants JP20K08695, JP20H03704 and JP23H02931), the Japan Agency for Medical Research and Development (grants JP22ek0109489, JP23ek0109672, JP23ek0109549, JP20am0101102 and JP21gm6310026), the Japan Science and Technology Agency (grant JPMJSP2123), the Takeda Science Foundation, the Uehara Memorial Foundation and the Hyogo Science and Technology Association. It was conducted in collaboration with researchers from Keio University, the National Cancer Center Research Institute, the National Center for Child Health and Development, and Japan Tissue Engineering Co. Ltd.
Original publication
S. Saito et al.: Gene-specific somatic epigenetic mosaicism of FDFT1 underlies a non-hereditary localized form of porokeratosis. The American Journal of Human Genetics (2024). DOI: 10.1016/j.ajhg.2024.03.017