The research group of Professor ONISHI Hiroshi in the Graduate School of Science at Kobe University has shed new light on the activation mechanism of photocatalysts for artificial photosynthesis upon doping with metal cations. Published in ACS Catalysis on April 24, 2015, their results challenge existing theories and should realize more efficient photocatalyst designs.
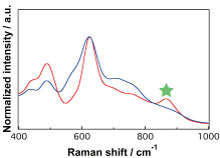
Much of the energy that we currently use originates from fossil fuels, which are not a sustainable resource. Numerous studies have investigated artificial photosynthesis as a method to produce chemical energy from alternative sources, such as sunlight and water. In particular, studies on photocatalysts to extract hydrogen gas, which is rare in the Earth’s atmosphere, have been conducted for the last 45 years and remain an active field.
In 2000, a research team in Japan discovered that sodium tantalite (NaTaO3 ) is a very efficient photocatalyst for splitting water to produce hydrogen. Moreover, the team found that doping NaTaO3 with metals such as strontium (Sr) and lanthanum (La) on the order of a few percent increased the hydrogen generation efficiency by an order of magnitude. Although this increased activity was thought to arise from some of the Na cations being substituted by similarly sized dopant metal cations, research was insufficient to verify this theory.
ONISHI’s research group synthesized Sr-doped NaTaO3 using two different methods. One resulted in the substitution of some of the Na with Sr, while the other resulted in the substitution of some of the Ta. Raman spectroscopy of the two compounds revealed that the latter with the substituted Ta cations acted as a more efficient photocatalyst, which is a paradigm shift that has led to the reexamination of the theory accepted for the last 15 years.
“I hope the mechanism that we revealed will become a key factor in the design of improved photocatalysts,” said ONISHI with anticipation.
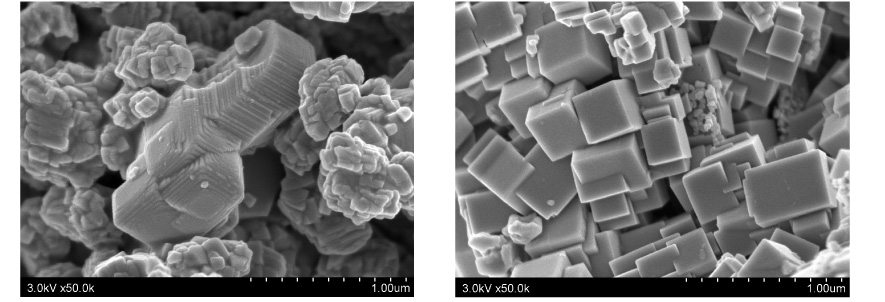
(Right) Doped NaTaO3 prepared by the hydrothermal method (2% Sr).
The two preparation methods result in very different particle size and shape.
Journal information
Title
“Electron–Hole Recombination Controlled by Metal Doping Sites in NaTaO3 Photocatalysts”
DOI
10.1021/acscatal.5b00484
Authros
Longjie An1, Hiroshi Onishi1
1 Graduate School of Science, Kobe UniversityJournal