A research group led by Professor MORI Yasuko (of the Division of Clinical Virology, Center for Infectious Diseases, Kobe University Graduate School of Medicine) has revealed that the HHV-6B glycoprotein complex gH/gL/gQ1/gQ2 is an effective vaccine candidate for human herpesvirus 6B (HHV-6B). There are still no methods to treat nor prevent HHV-6B infection, and this study represents the first attempt in the world at developing a vaccine.
This study was conducted through joint research between the following:
- Professor MATOZAKI Takashi et al. of the Division of Molecular and Cellular Signaling, Department of Biochemistry and Molecular Biology, Kobe University Graduate School of Medicine.
- Professor ITO Tomoo et al. of the Department of Diagnostic Pathology at Kobe University Hospital.
- Professor NISHIGORI Chikako of the Division of Dermatology, Department of Internal Related, Kobe University Graduate School of Medicine.
- Associate Professor AOISHI Taiki of the Vaccine Dynamics Project at BIKEN Innovative Vaccine Research Alliance Laboratories, Research Institute for Microbial Diseases of Osaka University.
- Professor SUZUKI Ryo of the Laboratory of Drug and Gene Delivery Research, Teikyo University.
- The Research Foundation for Microbial Diseases of Osaka University (BIKEN Foundation).
The results were published online in the American scientific journal ‘PLOS Pathogens’ on July 23.
Main points
- Human herpesvirus 6B (HHV-6B) is a pathogen that infects the vast majority of people when they are infants. It not only causes exanthem subitum (*1), with symptoms of a fever followed by a skin rash (roseola) but can also trigger severe complications with lasting after-effects such as febrile convulsions, encephalitis (brain inflammation) and encephalopathy.
- Methods to effectively prevent or treat HHV-6B infection have yet to be established. The infection rate is extremely high and great risks are posed by HHV-6B. It is hoped that the realization of a vaccine would enable infants to be inoculated against HHV-6B, resulting in widespread prevention of this virus.
- This research group previously discovered a HHV-6B glycoprotein complex that is an essential factor in HHV-6B infection. In this study, they utilized this complex as a vaccine antigen and analyzed its effectiveness.
- The research group inoculated mice with the purified virus antigen combined with immunostimulants known as adjuvants (*2), demonstrating that this induced effective immunity against HHV-6B. Furthermore, the combination with the adjuvants was also shown to induce cellular immunity (*3).
- These successful results are a big step towards the realization of a safe and effective vaccine for HHV-6B. It is hoped that this research can proceed to clinical trials.
Research Background
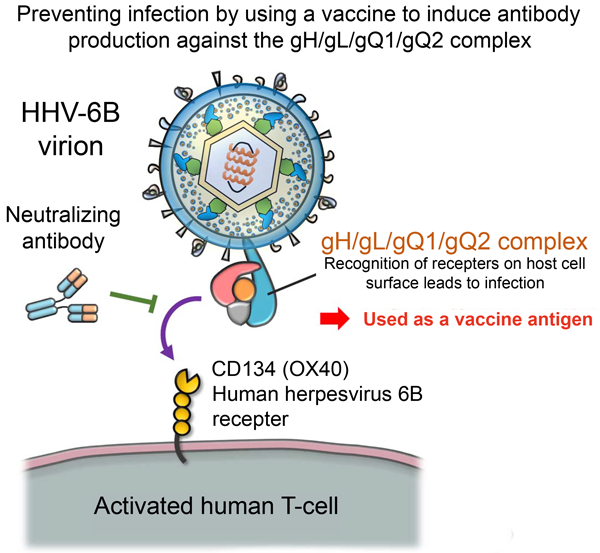
Human herpesvirus 6B (HHV-6B) is passed on to infants via the saliva of family members etc., causing exanthem subitum which has symptoms of a fever over 38°C followed by a rash all over the body (roseola). The overwhelming majority of people are infected with HHV-6B. The infection period is between 6 months and 2 years of age; this coincides with the diminishment of antibodies received from the mother.
In most cases, infants recover without experiencing any serious symptoms, however severe complications can occur. For example, it has been reported that in Japan, around 150 infants a year suffer encephalitis or encephalopathy, resulting in lasting aftereffects in around half of this number. Therefore, it is essential to develop a vaccine to protect infants from HHV-6B infection, as there is currently no established treatment nor preventative measures against the virus.
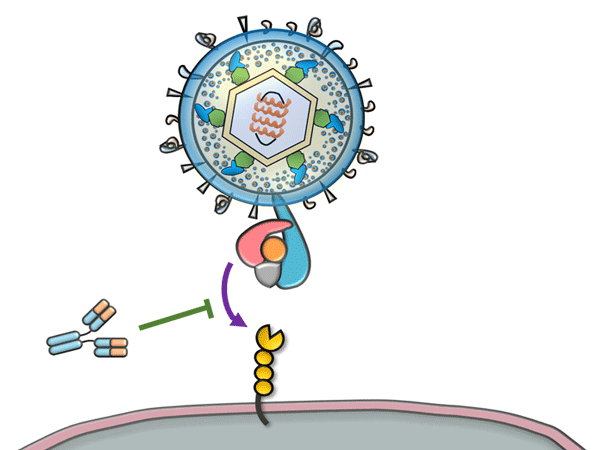
Previously, Professor Mori’s research group discovered the glycoprotein complex gH/gL/gQ1/gQ2, which is expressed on the HHV-6B virus’s surface. They also revealed that the interaction between this complex and CD314 (OX40), which is expressed on stimulated T-cells, is the key to infection (Figure 1). An antibody that targets the gH/gL/gQ1/gQ2 complex would be able to prevent HHV-6B infection. Therefore, the group is also conducting research into generating antibodies that can be used on humans from mice antibodies.
From this accumulated knowledge and experience came the following idea: an efficient immune response against HHV-6B infection could be achieved if inoculation with the gH/gl/gQ1/gQ2 complex induced immunity against the complex.
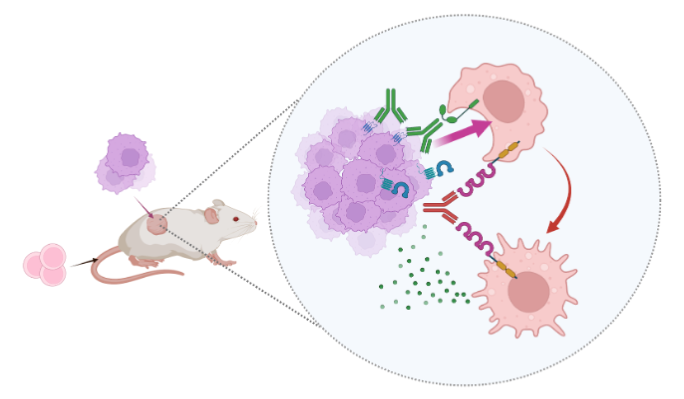
The researchers developed a vaccine based on the HHV-6B gH/gL/gQ1/gQ2 complex. (A Patent Application for the vaccine has been filed by the BIKEN Foundation and Kobe University (Patent Application No. 2017-509816)). They generated the gH/gL/gQ1/gQ2 complex via genetic modification techniques. This complex was utilized as the vaccine antigen and mice were inoculated with this in combination with an adjuvant, and immunity induction was analyzed (Figure 2).
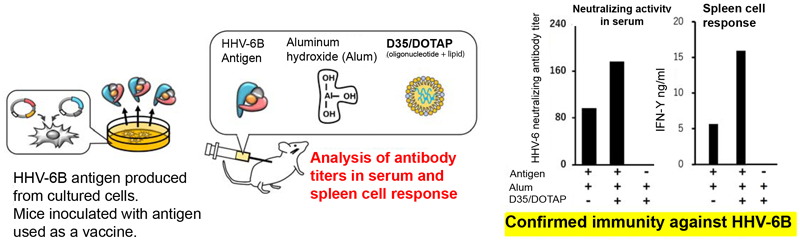
The HHV-6B gH/gL/gQ1/gQ2 complex is a complicated molecule constructed in a cell with four types of protein. A method was developed to grow this complex in a cultivated cell in which all the proteins are expressed at the same time. It was confirmed that the HHV-6B gH/gL/gQ1/gQ2 complexes generated using this method still retained their function of binding to their target receptor molecule, CD134 (OX40).
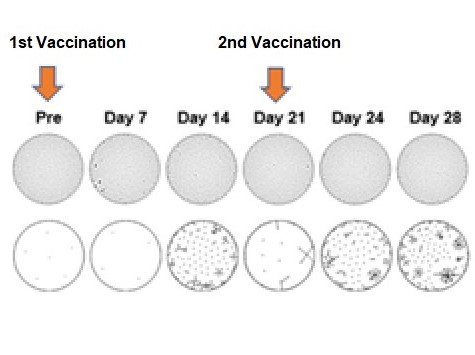
The complex was combined with the adjuvant aluminum hydroxide (abbreviated to Alum), which is widely used in current vaccines, and administered to mice in several doses. The immune response was analyzed; the results confirmed there were vaccine induced antibodies against the gH/gL/gQ1/gQ2 complex in the serum of the mice, and their serum had actually prevented HHV-6B from infecting the cells. Furthermore, it was shown that the glycoprotein complex itself had activated dendritic cells, inducing innate immunity (*4).
Furthermore, a vaccine with a combination of oligonucleotide D35 (which can induce cellular immunity) and its transporter, the DOTAP lipid, as adjuvants in addition to Alum was developed. This vaccine was demonstrated to induce an even stronger antibody response. Spleen cells were extracted from mice after the immunity experiments and the immune cell responses to the gH/gL/gQ1/gQ2 complex were investigated. The results showed a stronger response to the antigen in the group inoculated with the Alum/D35/DOTAP combination and confirmed that cellular immunity was induced. Additional analysis results revealed that CD4 T-cells were the main responders to the antigen.
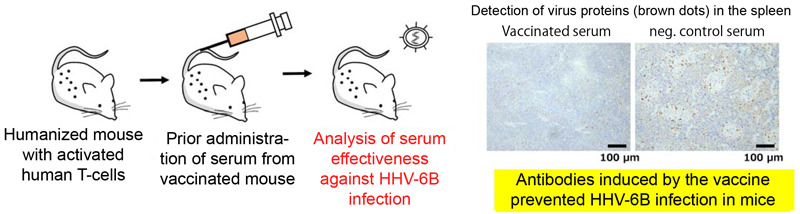
The researchers also investigated whether or not the induction of serum antibodies via inoculation with the gH/gL/gQ1/gQ2 complex actually prevented HHV-6B infection in animals (Figure 3). This experiment utilized immune cell-humanized mice (*5) to develop an animal model of HHV-6B infection. As a negative control, humanized mice were administered with serum from mice that had been given a vaccine containing only the adjuvant. The humanized mice were then injected with HHV-6B. The virus proliferated internally and many virus antigens were detected in the spleens of the negative control group.
On the other hand, the virus did not proliferate in humanized mice that received prior administration of serum from mice who were inoculated with the vaccine containing the gH/gL/gQ1/gQ2 complex. Also, there were hardly any virus antigens in the spleens of these humanized mice. This demonstrates that the induced immunity from the vaccine is efficient against HHV-6B infection in mice.
Further Developments
Effective treatment and preventative methods for HHV-6B infection have yet to be established despite the latent risks that it poses to the health of all infants. The results of this research represent a huge step towards the efficient prevention of HHV-6B infection with a vaccine. It was demonstrated that this vaccine, which used the gH/gL/gQ1/gQ2 complex as an antigen, efficiently induced an immune system response. Also, the vaccine is promising from a safety aspect as it is a subunit vaccine (*6) that does not contain other virus-derived molecules, aside from the complex. Currently, many infants are given a combined inoculation against four diseases called the DPT-IPV vaccine (D: Diphtheria, P: pertussis, T: tetanus and IPV: inactivated polio virus) at 3 months of age. It is hoped that HHV-6B inoculation could be added to this vaccine to prevent infants from contracting it.
After infection, HHV-6B remains latent inside its host for their entire life. It can be reactivated by conditions such as drug-induced hypersensitivity syndrome or a decline in immunity, and has been reported to trigger various illnesses. In particular, this as a problem when hematopoietic stem cell transplants are used to treat leukemia, leading to a high frequency of HHV-6B reactivation which can cause life-threatening encephalitis. The vaccine developed by this study, when combined with adjuvants, not only grants humoral immunity (*7) but can also induce cellular immunity. In other words, this vaccine can induce a strong immune response to HHV-6B. It is believed that it could also be used to suppress the HHV-6B infection in those undergoing hematopoietic stem cell transplants.
Next, the researchers will build upon these results, collect data on the effectiveness and safety of the vaccine and then proceed to clinical trials. They aim to bring a pioneering HHV-6B vaccine developed in Japan to the world.
Glossary
- 1. Exanthem subitum (roseola infantum)
- A viral infection that occurs in infants around 6 months old, which is characterized by a fever followed by a rash that develops after the temperature returns to normal. It is predominantly caused by HHV-6B infection, and sometimes by human herpesvirus 7 (HHV-7). The illness has a favorable prognosis for the majority, however, in rare cases severe complications such as febrile convulsions, encephalitis (brain inflammation) and encephalopathy can occur.
- 2. Adjuvant
- This is an immunostimulant that strengthens the body’s immune system functions. In vaccines, adjuvant(s) are utilized alongside antigen(s) in order to strengthen the immune system’s reaction to the antigen(s) and promote more effective immunity acquisition.
- 3. Cellular immunity
- An immune system response to a pathogen in which T-cells (that recognize specific foreign antigens) activate macrophages and cytotoxic T-cells to target and destroy the pathogen.
- 4. Innate immunity
- Immunity against a specific foreign pathogen etc. is activated via a widespread immune cell reaction to the specific pathogen.
- 5. Immune cell-humanized mouse model
- When human hematopoietic stem cells are inserted into a mouse with an immune cell deficit, the mouse retains the human immune cells. This is called a humanized mouse model. HHV-6B specifically infects human T-cells so it is not possible to infect a normal mouse with the virus. However, it has been confirmed that HHV-6B infection and proliferation can be induced inside the bodies of humanized mice- creating an animal model of HHV-6B.
- 6. Subunit vaccine
- A vaccine that only uses a part of the pathogen. An appropriate infection-related antigen that sticks out of the pathogen surface is selected in order to induce an effective immune response. It is thought that subunit vaccines can reduce the side effects of inoculation. However, subunit vaccines induce a weaker immune response compared to live vaccines (that utilize weakened pathogens) so effective use of the adjuvant is an issue.
- 7. Humoral immunity
- An immune system response in which the T cells (that recognize specific foreign antigens) stimulate B cell antibody production. These antibodies are distributed via the blood and lymph fluid where they bind to the target pathogen in order to neutralize or eliminate it.
Acknowledgements
This research was made possible with funding from the following:
- Acceleration Transformative research for Medical Innovation (ACT-MS) from the Japan Agency for Medical Research (AMED) (Lead Researcher Mori Yasuko)
- Health and Labour Sciences Research Grants (Japan) from the Ministry of Health, Labour and Welfare (MHLW), for Research on Development of New Drugs and Research on Emerging and Re-emerging Infectious Disease (Lead Researcher: Mori Yasuko).
- A Grant-in-Aid for JSPS Fellows from the Japan Society for the Promotion of Science (recipient: Wang Bochao).
Journal Information
- Title
- “Tetrameric glycoprotein complex gH/gL/gQ1/gQ2 is a promising vaccine candidate for human herpesvirus 6B”
- DOI
- 10.1371/journal.ppat.1008609
- Authors
- Bochao Wang1, Kouichi Hara1, Akiko Kawabata1, Mitsuhiro Nishimura1, Aika Wakata1, Lidya Handayani Tjan1, Anna Lystia Poetranto1, Chisato Yamamoto1, Yasunari Haseda2, Taiki Aoshi2,3, Lisa Munakata4, Ryo Suzuki4, Masato Komatsu5, Ryuko Tsukamoto5, Tomoo Itoh5, Chikako Nishigori6, Yasuyuki Saito7, Takashi Matozaki7, Yasuko Mori1
- Division of Clinical Virology, Center for Infectious Diseases, Kobe University Graduate School of Medicine
- Vaccine Dynamics Project at BIKEN Innovative Vaccine Research Alliance Laboratories, Research Institute for Microbial Diseases of Osaka University
- BIKEN Center for Innovative Vaccine Research and Development, The Research Foundation for Microbial Diseases of Osaka University
- Laboratory of Drug and Gene Delivery Research, Teikyo University
- Department of Diagnostic Pathology, Kobe University Hospital
- Division of Dermatology, Department of Internal Related, Kobe University Graduate School of Medicine
- Division of Molecular and Cellular Signaling, Department of Biochemistry and Molecular Biology, Kobe University Graduate School of Medicine
- Journal
- PLOS Pathogens