An international collaborative research group consisting of members from 7 institutions has developed a method of determining which amphibious species (types of frog, newt and salamander) inhabit an area. They achieved this by amplifying extra-organismal DNA (environmental DNA) found in the water so that it could be analyzed. This DNA ends up in the water because it is expelled from the amphibian’s body along with mucus and excrement. The research group included Postdoctoral Researcher SAKATA K. Masayuki and Professor MINAMOTO Toshifumi (Kobe University), Associate Professor KURABAYASHI Atsushi (Nagahama Institute of Bio-Science and Technology), NAKAMURA Masatoshi (IDEA Consultants, Inc.) and Associate Professor NISHIKAWA Kanto (Kyoto University).
The developed technique will resolve some of the issues with conventional methods such as capture and observational surveys, which require a specialist surveyor who can visually identify species. Conventional surveys are also prone to discrepancies in results caused by environmental factors such as climate and season. It is hoped that the new method will revolutionize species monitoring, as it will enable anyone to easily monitor the amphibians that inhabit an area by collecting water samples. These research results were published in the online international academic journal Metabarcoding and Metagenomics on Monday February 21st at 4pm JST.
Main points
- Monitoring biodiversity is vital for the conservation of natural ecosystems as a whole. In particular, the importance of monitoring amphibians is increasing, as the number of individuals is declining significantly.
- However, amphibians are nocturnal, young (e.g. tadpoles) and adults live in different habitats, and specialist knowledge is required to capture individuals and identify their species. These issues that make it difficult to accurately monitor amphibians in a standardized way, thus the results of individual surveys often contradict each other.
- Environmental DNA (eDNA) is extra-organismal DNA that has been released into the environment. In recent years, eDNA analysis techniques have been used to monitor species. Methods for monitoring fish species in particular have been established and are now commonplace. However, a standardized analysis method for amphibians has yet to be established. In order to contribute towards its development, this research group collected and analyzed the DNA of amphibia to come up with an analysis method for investigating which amphibious species inhabit a surveyed area.
- First of all, the researchers designed multiple methods for analyzing the eDNA of amphibians and evaluated their performance to identify the most effective method. Next, they conducted parallel monitoring of 122 sites in 10 farmlands across Japan using the developed eDNA analysis method and conventional methods (capture surveys using a net and observation surveys).
- The newly developed method was able to detect all three orders of amphibian: Caudata (the newt and salamanders), Anura (the frogs), and Gymnophiona (the caecilians). Furthermore, this novel eDNA analysis method was able to detect more species across all field study sites than the conventional method-based surveys, indicating its effectiveness.
Research Background
Amphibian biodiversity is continuing to decline worldwide and collecting basic information about their habitats and other aspects via monitoring is vital for conservation efforts. Traditional methods of monitoring amphibians include visual and auditory observations, and capture surveys. However, amphibians tend to be small in size and many are nocturnal. The success of surveys varies greatly depending on the climate and season, and specialist knowledge is required to identify species. Consequently, it is difficult to monitor a wide area and assess habitats. The last decade has seen the significant development of environmental DNA analysis techniques, which can be used to investigate the distribution of a species by analysing external DNA (environmental DNA) that is released into the environment along with an organism’s excrement, mucus and other bodily fluids.
The fundamentals of this technique involve collecting water from the survey site and analysing the eDNA contained in it to find out which species inhabit the area. In recent years, the technique has gained attention as a supplement for conventional monitoring methods. Standardized methods of analysis have already been established for other species, especially fish, and diversity monitoring using eDNA is becoming commonplace.
However, eDNA monitoring of amphibians is still in the developmental stage. One reason for this is that the proposed eDNA analysis method must be suitable for the target species or taxonomic group, and there are still issues with developing and implementing a comprehensive method for detecting amphibians. If such a method could be developed, this would make it possible for monitoring to be conducted even by people who do not have the specialized knowledge to identify species nor surveying experience. Hopefully, this would be established as a unified standard for large-scale monitoring surveys, such as those on a national scale. This research group’s efforts to develop and evaluate analysis methods will hopefully lay the foundations for eDNA analysis to become a common tool for monitoring amphibians, as well as fish.
Research Methodology
Design and Evaluation of a Universal Primer for Amphibia
The researchers obtained a total of 1,034 amphibian DNA sequences from an online open database. They located the DNA regions where the base sequence is the same across different species and designed five analytical systems in the mitochondrial 16SrRNA region that could comprehensively amplify amphibian DNA. After that, they used a computer to investigate the various characteristics of each set, including the ease of amplifying amphibian DNA, the likelihood of non-amphibian DNA being amplified as well (i.e. the specificity), and whether they could reliably determine the species (i.e. the species-level resolution). In addition, they conducted experiments using DNA obtained from individual amphibians to confirm that the DNA of various species in the class could be amplified. The results revealed that all of the assays could amplify the DNA well. From the findings of all these prestudies, the researchers selected the assay that had the best specificity and species-level resolution and then carried out a field study to evaluate its efficiency as a monitoring tool.
Validating efficiency thorough field survey-based comparisons with conventional methods
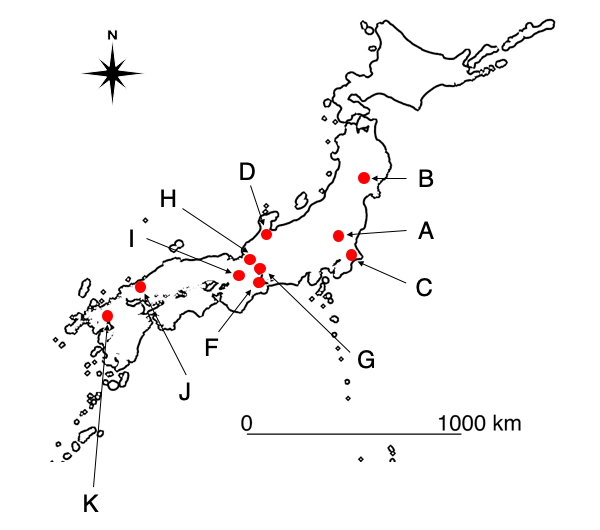
The red dots indicate the regions where the surveys were conducted.
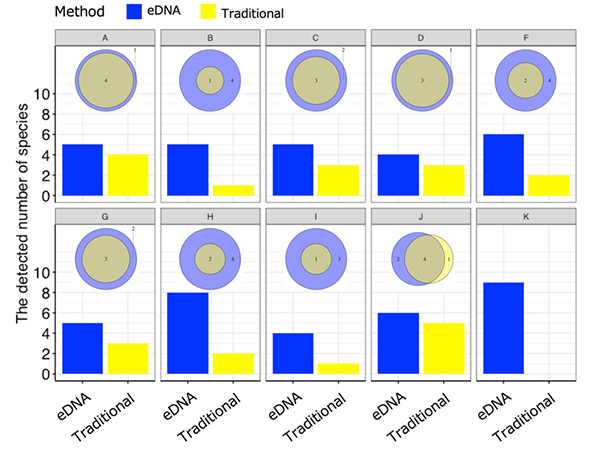
From June to September 2019, field studies were carried out at 122 sites across 10 agricultural ecosystems (paddy field ecosystems) in Japan in order to check the suitability and efficiency of the designed assay for field monitoring (Figure 1). In the field surveys, both eDNA analysis and conventional method-based surveys (capture/observations) were conducted in the ponds and agricultural waterways at each site, and the number and composition of the detected amphibia were compared. As shown in figure 2, the number of species detected through eDNA analysis was greater than the number detected through conventional methods across all field study sites. This consequently demonstrates that the eDNA analysis method using amphibian assays developed by this research group is highly effective for field monitoring.
Further Developments
- The researchers developed an analysis method that can comprehensively detect amphibian DNA. The eDNA monitoring system using this method could also contribute towards rapid surveying of endangered and important species.
- Unlike conventional survey methods like capture and observations, eDNA analysis is not easily affected by environmental factors, such as season and climate. Therefore, it is a stable way to detect amphibians and it is hoped that it will become the standardized method for large-scale surveys, such as nationwide monitorings.
- A collection of reference data to determine which species the amplified DNA originates from would enable this technique to be applied more efficiently. This would significantly contribute towards the monitoring of amphibians as the more reference data is collected, the greater the range of countries and regions where this technique can be utilized.
Glossary
- Environmental DNA (eDNA)
- DNA originating from organisms that is found in the environment, such as in the water or on the ground. By analyzing environmental DNA using PCR, researchers can comprehensively identify species that inhabit an area, even if they no longer live there. This in turn enables them to estimate and gain an understanding of the target species’ distribution. Environmental DNA surveys are used in a wide range of research fields, including species conservation, ecology, taxonomy, microbiology and palaeontology. Prior research using eDNA analysis has been carried out on fish species, and eDNA analysis is becoming a common tool for monitoring organisms. However, such methods for monitoring amphibians are still a work in progress, and a detection system that can be used to conduct thorough biodiversity surveys has yet to be established.
- Metabarcoding (of eDNA)
- A method of detecting multiple species simultaneously. A universal primer consists of the nucleotide sequence regions that are the same for the target taxa. Using this universal primer, the collated DNA of multiple species that includes those in the target taxa can be amplified using PCR. Next, the sequences of the amplified sections are comprehensively determined using a massively parallel sequencing. Species can be identified by matching the sequences to those registered in a database.
Journal Information
- Title
- “Development and evaluation of PCR primers for environmental DNA (eDNA) metabarcoding of Amphibia”
- DOI
- 10.3897/mbmg.6.76534
- Authors
- Masayuki K. Sakata, Mone U. Kawata, Atsushi Kurabayashi, Takaki Kurita, Masatoshi Nakamura, Tomoyasu Shirako, Ryosuke Kakehashi, Kanto Nishikawa, Mohamad Yazid Hossman, Takashi Nishijima, Junichi Kabamoto, Masaki Miya, Toshifumi Minamoto
- Journal
- Metabarcoding and Metagenomics