An international research collaboration, including Professor IIJIMA Kazumoto et al. (of the Department of Pediatrics, Kobe University Graduate School of Medicine) has revealed that NPHS1 is a disease-susceptibility gene for steroid-sensitive nephrotic syndrome in children. The NPHS1 gene encodes nephrin, a component protein for the renal glomerulus slit diaphragm, which prevents protein from being passed in the urine. It is expected that these successful results will contribute towards understanding of the underlying mechanism and the development of new treatments for childhood nephrotic syndrome.
Main researchers who contributed towards these findings included those from the following institutions:
- Kobe University Graduate School of Medicine’s Department of Pediatrics: Professor IIJIMA Kazumoto, Project Professor NOZU Kandai, Assistant Professor YAMAMURA Tomohiko, Project Assistant Professor NAGANO China and Project Assistant Professor HORINOUCHI Tomoko;
- National Center for Global Health and Medicine’s Genome Medical Science Project (Toyama): including Project Leader TOKUNAGA Katsushi and Specially Appointed Researcher Xiaoyuan Jia;
- Specially-appointed lecturer HITOMI Yuki’s research group in the Department of Microbiology at Hoshi University School of Pharmacy and Pharmaceutical Sciences;
- Associate Professor Matthew G. Sampson (of the Department of Medicine-Nephrology, Boston Children’s Hospital);
- Professor Pierre Ronco (Department of Nephrology, Sorbonne University);
- Prodiatrics, Duke University Medical Center);
- Professor Hae Il Cheong (Department of Pediatrics, Seoul National University Children’s Hospital);
- Professor Kyuyong Song (Department of Biochemistry and Molecular Biology, University of Ulsan College of Medicine).
The results of this study were published online in the international scientific journal ‘Kidney International’ on June 13.
Main points
- Nephrotic syndrome (*1) causes excessive amounts of protein to be passed in the urine, resulting in severely low levels of protein in the blood. It is the most commonly occurring childhood chronic kidney disease. In Japan, it is both a designated intractable disease and a specific pediatric chronic disease. The cause has not been illuminated.
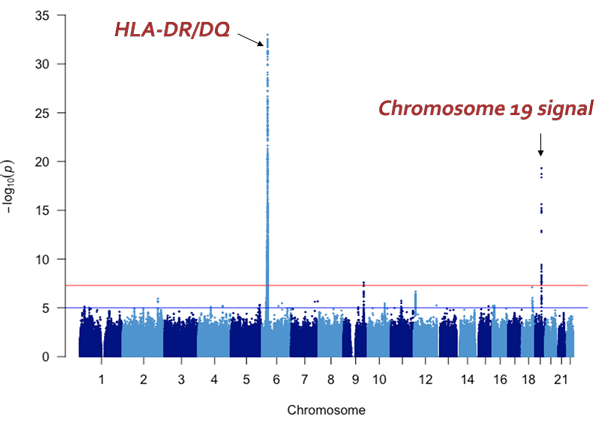
- The majority of cases in children are steroid-sensitive nephrotic syndrome (*2), which can be ameliorated with steroids. It is believed that an immunological trigger, such as an infection, stimulates the syndrome’s occurrence in people who have some kind of genetic susceptibility (disease-susceptibility gene (*3)). It is understood that HLA-DR/DQ is a disease-susceptibility gene for this syndrome, however susceptibility genes outside the HLA have not been illuminated.
- A Genome-Wide Association Study (GWAS, *4) was conducted to compare the Single Nucleotide Polymorphisms (SNPs) across all regions of the genome in Japanese patients with childhood steroid-sensitive nephrotic syndrome with those of healthy people. A trans-ethnic international meta-analysis (*5) was then conducted, and it revealed that NPHS1 is a disease-susceptibility gene.
- It is hoped that these findings will contribute towards illuminating the underlying mechanism behind childhood nephrotic syndrome and the development of new treatments.
Research Background
Childhood nephrotic syndrome is the most common chronic kidney disease affecting children; in Japan it occurs at a yearly rate of 6.49 children out of every 100,000 (approximately 1,000 cases countrywide). It is a disease with unclear causes in which excessive amounts of protein are passed in the urine, resulting in severely low levels of protein in the blood. In Japan, it has been classified as both a specific pediatric chronic disease and a designated intractable disease. Between 80-90% of childhood nephrotic syndrome cases are steroid-sensitive nephrotic syndrome, meaning that they can be sent into complete remission through steroid treatment. However, about 20% of patients experience repeated relapses even in adulthood. There is a strong demand to illuminate the disease’s causes and pathology, and utilize this knowledge to develop a definitive treatment method.
The majority of steroid-sensitive nephrotic syndrome cases are multifactorial. It is thought to occur due to a combination of some kind of genetic factor (disease-susceptibility gene) and an immunological trigger, such as an infection. Professor Iijima et al.’s research up until now has revealed that HLA-DR/DQ is a disease-susceptibility gene, however susceptibility genes outside the HLA region have yet to be illuminated.
Research Findings
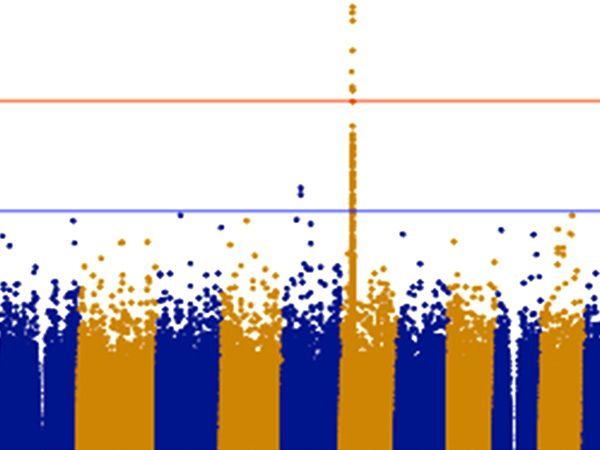
The vertical axis shows the P values (-log10) for each SNP, obtained from the GWAS of healthy participants and patients with steroid-sensitive nephrotic syndrome. The position of the chromosomes is plotted on the horizontal axis; identifying the genome-wide significant (P< 5x10-8) signal from chromosome 19.
The research group has so far collected genomic DNA from around 1,300 patients with childhood nephrotic syndrome, with the cooperation of pediatric nephrology specialists across Japan. For this particular research, they used samples from 987 of the above cases that were childhood steroid-sensitive nephrotic syndrome with samples from 3,206 healthy Japanese donors as a control. GWAS was performed using the SNP array ‘Japonica’, which is the most appropriate for carrying out this genetic test on Japanese individuals. From these results, variants in the NPHS1-KIRREL2 region on chromosome 19 (19q13.12) with genome-wide significance were identified (Figures 1 and 2). These variants are outside the HLA-DR/DQ region.
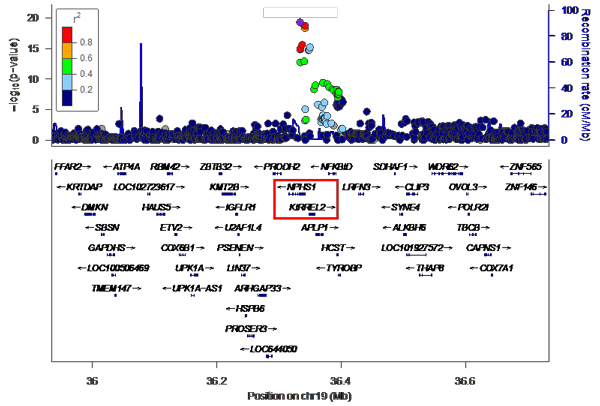
This is an enlarged version of part of Figure 1 with the genetic regions narrowed down. It can be seen that the NPHS1-KIRREL2 region (indicated by the red box) on chromosome 19 has genome-wide significance.
Trans-ethnic replication studies into these multiple variants in the NPHS1-KIRREL2 region were conducted across various populations, including Korean, South Asian, African, European, Hispanic and Maghrebian (Northwest African). The investigation covered patients with steroid-sensitive nephrotic syndrome (1,063 people in total) and healthy counterparts of the same ethnicity (19,729 people in total). The results were replicated in the Korean, South Asian and African datasets. An international meta-analysis including the Japanese cohort also illuminated the significance of these multiple variants in NPHS1.
Subsequently, the relationship between these multiple variants in NPHS1 and NPHS1 mRNA expression in the glomerulus was investigated. The NPHS1 mRNA expression originating from chromosomes with haplotypes (*7) containing all the risk variants was low, revealing that these variants play a role in NPHS1 mRNA regulation.
NPHS1 is the gene responsible for encoding nephrin (*6). Nephrin is the most important protein for the formation of the renal glomerulus slit diaphragm, which acts as a barrier against urinary proteins (Figure 3). It is widely known that NPHS1 is the gene that causes congenital nephrotic syndrome Finnish type (*8), a rare Mendelian genetic disorder.
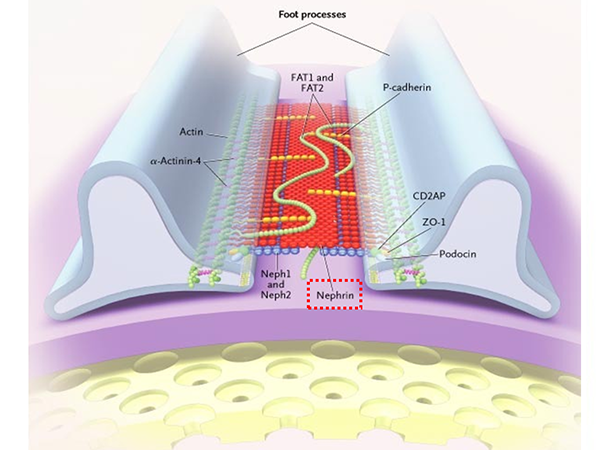
The renal glomerulus slit diaphragm is located in-between the podocyte foot processes and functions as a barrier to prevent protein entering the urine. Nephrin (indicated by the red box), is the most important component protein for the formation of the renal glomerulus slit diaphragm and NPHS1 is the gene that encodes nephrin.
(Slightly altered version of: Tryggvason K et al. Hereditary Proteinuria Syndromes and Mechanisms of Proteinuria. N Engl J Med 2006; 354:1387-1401, Fig.5. Components of the Slit-Diaphragm Protein Complex That Form a Porous Slit-Diaphragm Filter.)
This research study revealed that NPHS1 is also a disease-susceptibility gene for steroid-sensitive nephrotic syndrome, the most commonly occurring multifactorial kidney disorder in children.
This research represents an important milestone in our understanding of the genetics behind steroid-sensitive nephrotic syndrome’s mechanism. It will also bring about a paradigm shift in the field of nephrology. These results will contribute towards the illumination of the pathology of childhood nephrotic syndrome. It is hoped that such knowledge could be applied to the development of new treatment methods.
Futher Developments
Next we will investigate the link between HLA and nephrin. We aim to develop more effective, safe treatment and prevention methods for steroid-sensitive nephrotic syndrome while illuminating the causes and pathology behind the disease.
Glossary
- 1. Nephrotic Syndrome
- Those with nephrotic syndrome pass too much protein in their urine, causing the amount of protein in the blood to decrease (Hypoproteinemia). This results in swelling (edema), particularly around the eyes and in the feet and ankles. It is called Primary Nephrotic Syndrome in cases where the cause is unclear. In Japan, it has been classified as one of the designated intractable diseases and specific pediatric chronic diseases.
- 2. Steroid-sensitive nephrotic syndrome
- A type of nephrotic syndrome, it can go into complete remission (i.e. the symptoms can be cured) within 4 weeks of daily steroid administration. However, repeated relapses are common.
- 3. Disease-susceptibility gene
- It is believed that multifactorial disorders are caused by a combination of genetic factors and environmental factors. A gene that leads to the onset of a specific multifactorial disease is known as a disease-susceptibility gene.
- 4. Genome-wide Association Study (GWAS)
- GWAS is a method of statistically investigating the relationship between Single Nucleotide Polymorphism (SNP) frequency and illness or quantitative traits, using an SNP array to determine gene sequence. An SNP array can genotype between 500,000 to 1 million sites among over 10 million SNP sites that cover almost the entire human genome.
- 5. Meta-analysis
- Collating and consolidating research data from multiple independent sources and statistically analyzing it.
- 6. Nephrin
- A component protein that plays an important role in the formation of the renal glomerulus slit diaphragm, which acts as a barrier against urinary proteins.
- 7. Haplotype
- When two of the same chromosome are inherited each from one parent, they generate one homologous chromosome. A haplotype is a cluster of alleles on a chromosome (haploid) that were inherited from one parent.
- 8. Congenital nephrotic syndrome Finnish type
- A type of nephrotic syndrome that occurs within 3 months after birth due to a mutation in the NPHS1 gene, which codes nephrin. It is a Mendelian genetic disorder (monogenetic disorder) and is inherited in an autosomal recessive manner.
Acknowledgements
This research received funding from the following:
- The Japan Agency for Medical Research and Development (AMED)’s Tailor-Made Medical Treatment with the BioBank Japan Project.
- AMED’s Platform Program for Promotion of Genome Medicine’s ‘Identification of disease susceptible genes and drug sensitive genes in childhood nephrotic syndrome’.
- AMED’s Platform Program for Promotion of Genome Medicine (P3GM)’s Advanced Genome Research and Bioinformatics Study to Facilitate Medical Innovation (GRIFIN):‘Development and application of bioinformatics methods to facilitate the detection of genes associated with multifactorial disorders based on large-scale whole genome sequencing data of Japanese individuals’
- The Japan Society for the Promotion of Science’s Fund for the Promotion of Joint International Research (Fostering Joint International Research (B)): ‘Joint International Research for identification of disease-susceptible genes and drug-sensitive genes in childhood nephrotic syndrome’
The researchers would also like to thank pediatric nephrology specialists nationwide for their cooperation.
Journal Information
- Title
- “Common risk variants in NPHS1 and TNFSF15 are associated with childhood steroid-sensitive nephrotic syndrome”
- DOI
- 10.1016/j.kint.2020.05.029
- Authors
- Jia X*, Yamamura T*, Gbadegesin R*, McNulty M*, Song K, Nagano C, Hitomi Y, Lee D, Aiba Y, Khor SS, Ueno K, Kawai Y, Nagasaki M, Noiri E, Horinouchi T, Kaito H, Hamada R, Okamoto T, Kamei K, Kaku Y, Fujimaru R, Tanaka R, Shima Y, The Research Consortium on Genetics of Childhood Idiopathic Nephrotic Syndrome in Japan, Baek J, Kang HG, Ha IS, Han KH, Yang EM, Korean Consortium of Hereditary Renal Diseases in Children, Abeyagunawardena A, Lane B, Chryst-Stangl M, Esezobor C, Solarin A, Midwest Pediatric Nephrology Consortium (Genetics of nephrotic syndrome study group), Dossier C, Deschênes G, NEPHROVIR, Vivarelli M, Debiec H, Ishikura K, Matsuo M, Nozu K, Ronco P, Cheong HI, Sampson MG, Tokunaga K#, Iijima K#(* Jointly First author、# Jointly responsible author)
- Journal
- Kidney International