A research group including Kobe University’s Professor TAKUMI Toru (also a Senior Visiting Scientist at RIKEN Center for Biosystems Dynamics Research) and Assistant Professor TAMADA Kota, both of the Physiology Division in the Graduate School of Medicine, has revealed a causal gene (Necdin, NDN) in autism model mice that have the chromosomal abnormality*1 called copy number variation*2.
The researchers hope to illuminate the NDN gene’s molecular mechanism in order to contribute towards the creation of new treatment strategies for developmental disorders including autism.
These research results were published in Nature Communications on July 1, 2021.
Main points
- The research group identified Ndn as a causal gene of autism by conducting a screening based on synaptic expression in an animal model of the disorder (15q dup mouse).
- The Ndn gene regulates synapse*3 development during the developmental stage.
Research Background
Even though the number of patients diagnosed with autism (autism spectrum disorder) has been greatly increasing, many aspects of this developmental disorder are still not well understood. Its causes are divided into genetic factors and environmental factors. Within these genetic factors, particular copy number variations have been found in autistic patients; for example, chromosome 15q11-q13 duplication. These abnormalities in the 15q11-q13 region are divided into maternally derived and paternally derived chromosomal duplication cases. It is understood that the Ube3a gene drives maternally derived chromosomal duplication. However, it is not known which gene is vital for paternally derived duplication.
This research group previously succeeded in developing a mouse model of 15q11-q13 duplication (15q dup mouse). Using this mouse model, they identified numerous abnormalities in paternally derived chromosomal duplication cases, including autism-like behaviors, and abnormalities in dendritic spine*4 formation. However, the researchers were unable to identify which gene is responsible for autism-like behavior because this region contains many non-coding RNA molecules and genes that code proteins.
Research Methodology
In 15q dup mice, there is a great number of genes as the duplication extends to the 6Mb region. Previous research showed that behavioral abnormalities were not induced by maternally derived chromosomal duplication, therefore around 2Mb was excluded. As for the remaining 4Mb, the researchers first created a new 1.5Mb duplication mouse model and investigated behavior abnormalities. From the results, they were unable to identify any autism-like behavioral abnormalities in 1.5Mb duplication mice. Consequently, the researchers excluded this 1.5Mb, leaving them with three protein-encoding genes as possible candidates.
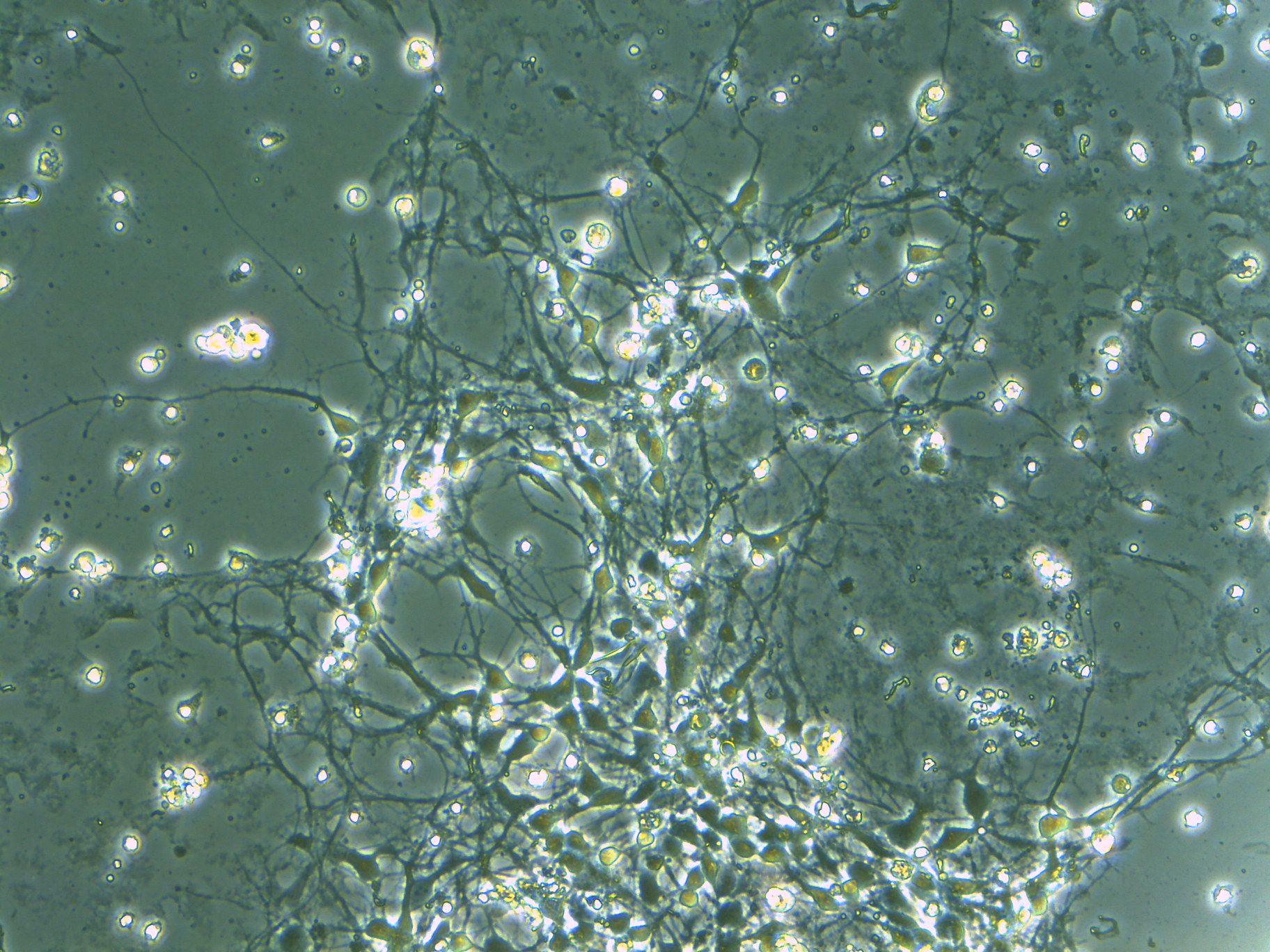
Next, these three genes were individually introduced into the cerebral cortex of mice via in utero electroporation*5. The researchers measured the spine turnover rate (formation and elimination of dendritic spines over a 2 day period) in vivo using a two-photon microscope*6 and discovered that the number of spines drastically increased when the Ndn gene was introduced (Figure 1A-C). Furthermore, morphology classification of these spines indicated that the majority were immature. This reveals that the Ndn gene regulates the formation and maturation of dendritic spines during the developmental stage (Figure 1D).
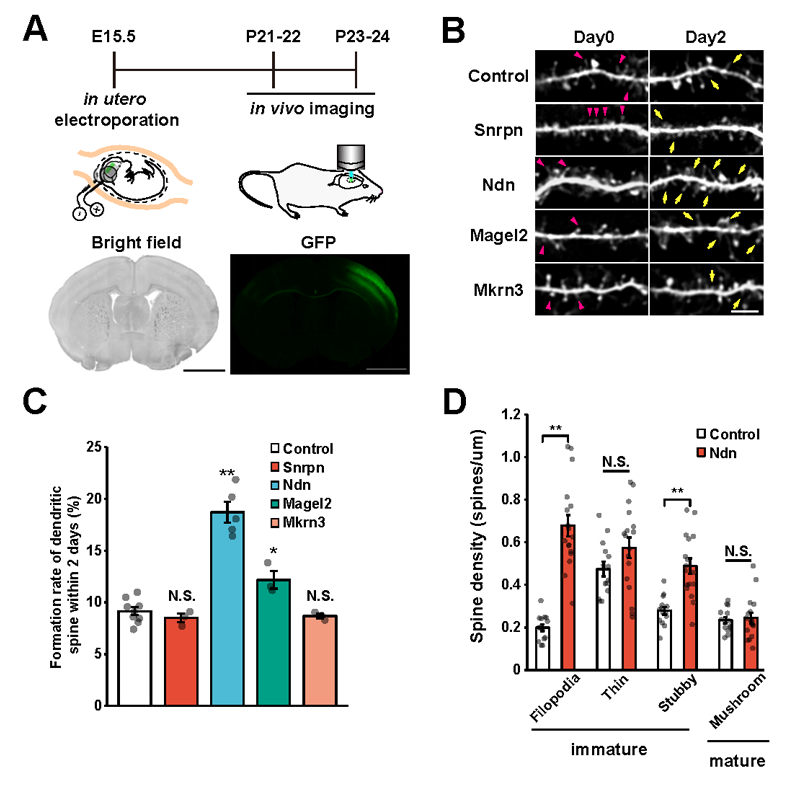
B: Each target gene was separately introduced into the cerebral cortex and dendritic spine dynamics were tracked for a period of 2 days (on the 21st and 22nd days after birth). Yellow arrows indicate newly formed spines and red arrowheads indicate spine elimination.
C: The quantified results of B in graph form.
D: Spine morphology classification data on the spines that formed when Ndn was introduced. Filopedia, a type of immature spine formation, significantly increased upon Ndn introduction.
Using CRISPR-Cas9*7, the researchers subsequently removed the one copy of the Ndn gene from the 15q dup mouse model to generate mice with a normalized genomic copy number for this gene (15q dupΔNdn mouse). Using this model, they demonstrated that the abnormalities observed in 15q dup mice (abnormal spine turnover rate and decreased inhibitory synaptic input) could be ameliorated (Figure 2).
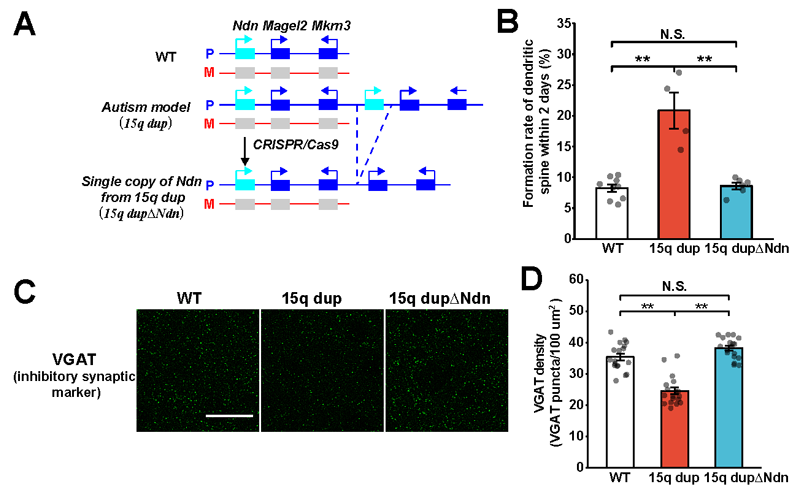
B: Dendritic spine formation rate in 15q dupΔNdn mice.
C and D: Quantification of inhibitory synapses.
Lastly, the researchers investigated whether the previously observed autism-like behaviors in 15q dup mice (including increased anxiety in a new environment, reduced sociability and increased perseveration) were evident in 15q dupΔNdn mice. They showed that in the majority of behavioral test results for 15q dupΔNdn mice, abnormal behaviors related to sociability and perseveration were ameliorated (Figure 3).
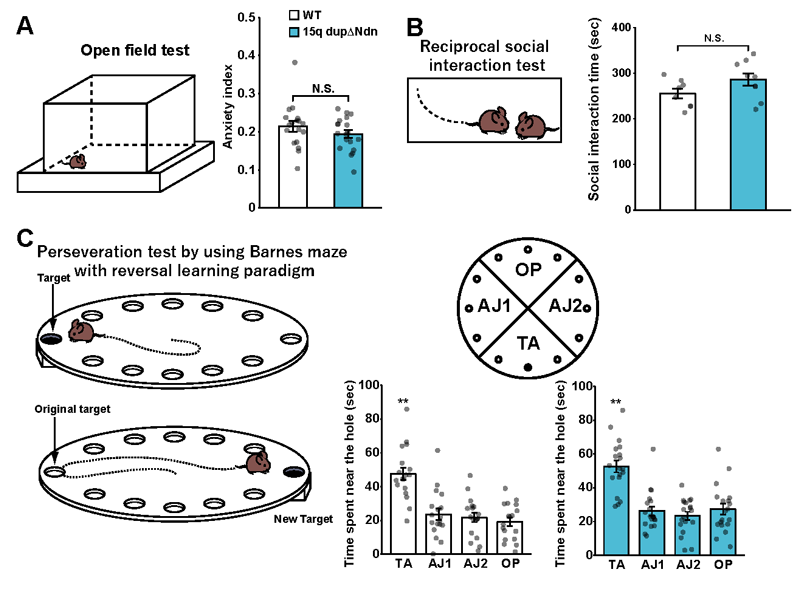
B: The results of the Social Interaction Test. Two mice that have never encountered each other before are placed in the same box. Sociability is evaluated by observing how long it takes them to interact and their physical contact. In the research group’s studies up until now, 15q dup mice’s sociability during this test was lower than that of normal mice.
C: The results of the Reverse Learning Test using a Barnes maze. A Barnes maze is a circular plate with 12 holes in it where only one of the holes is the ‘target’. The target hole has a box below it that the mouse can enter. A bright light is positioned above the circular plate. Mice generally dislike bright light and prefer dark places, so if a mouse is put on the plate, it will look for somewhere to hide. Using this characteristic, the mouse can be made to learn the target hole’s location over a period of days (C, upper illustration). Once the mouse has learned the target’s location correctly, the target’s location is reversed and relearned (C, lower illustration). It is understood that normal mice learn the new target location comparatively quickly compared to 15q dup mice who are slow to learn the new target, and persist in going to the original target’s location (perseveration). For the learning test, the target is removed and learning is evaluated by observing which hole(s) the mouse goes to and how long it stays there (Figure 3- right: TA= the new target hole and the two holes on either side. AJ1, AJ2: The three holes in each adjacent sector, OP: The previously learned target, and the two holes on either side.).
Further Research
This research study revealed that in 15q dup autism model mice, the NDN gene does not only play an important role in autism-like behaviors, but also affects aspects such as excitation/inhibition imbalance in synaptic dynamics and the cerebral cortex. Next, the research team hopes to clarify the NDN gene’s functions. By artificially regulating these functions or identifying and controlling their downstream factors, the researchers hope to understand the onset mechanism of developmental disorders like autism, and develop new treatment strategies.
Glossary
*1 Chromosomal abnormality
Chromosomes are structures that contain genes and are located inside the cell nucleus. Abnormalities such as duplications or deletions in specific regions of the chromosome are often seen in those with autism.
*2 Copy number variation
A chromosomal abnormality where the number of copies of a region on the genome is abnormal (duplications or deletions). Normally there should be 2 copies (diploid). If there are 3 copies or just one copy, then this is a copy number variation.
*3 Synapse
A junction between two nerve cells (neurons), which is involved in neuronal activities such as transmitting signals.
*4 Dendritic spine
These are protrusions on the dendrites of excitable neurons that receive input from synapses.
*5 in utero electroporation
A method of introducing outside DNA into the brain. DNA is injected through the wall of the uterus and is induced to enter the cells using electric waves.
*6 Two-photon microscope
A microscope with powerful laser, which enables the brains of mice to be observed while they are still alive. In this study, it was used to track changes in dendritic spines over a 2 day-period.
*7 CRISPR-Cas9
Technology that enables a specific region of a genome to be precisely cut and altered. This genome editing method won the 2020 Nobel Prize in Chemistry. In this study, it was used to remove the Ndn gene from the genome.
Acknowledgements
This research was partially funded by KAKENHI grants from the Japan Society for the Promotion of Science (JSPS), including in the innovative area entitled: 'Dynamic regulation of brain function by Scrap & Build system', and the Takeda Science Foundation among others.
Journal Information
Title
“Genetic dissection identifies Necdin as a driver gene in a mouse model of paternal 15q duplications”
DOI
10.1038/s41467-021-24359-3
Authors
Kota Tamada, Keita Fukumoto, Tsuyoshi Toya, Nobuhiro Nakai, Janak R Awasthi, Shinji Tanaka, Shigeo Okabe, Francois Spitz, Fumihito Saitow, Hidenori Suzuki, Toru Takumi
Journal
Nature Communications