Membrane technologies contribute towards advances in tackling global environmental issues and improving people’s quality of life. Examples include water purification, desalination, industrial wastewater treatment, the separation and collection of carbon dioxide (CO2) from exhaust gas, and energy-saving in petrochemical plants. As the only research institute for membrane science and technology in Japan, the Research Center for Membrane and Film Technology at Kobe University promotes academic–industrial partnerships and international joint research that aims to achieve the Sustainable Development Goals (SDGs).
Today, we interviewed Professor YOSHIOKA Tomohisa, a core member working on joint research with domestic and international institutes and industries, about his research themes and the aims of his international joint research projects.
Utilizing Molecular Simulations
Have you always studied membrane science and technology?
Professor Yoshioka:
I studied adsorption until I completed my doctorate. Well-known examples of materials that adsorb and separate molecules include activated carbon and silica gel. The latter is applicable to dehumidification. I conducted actual experiments and computer simulations to develop and assess adsorbents, which are porous materials with extremely tiny pores. In fact, adsorption and membranes have a lot in common. For example, they both utilize porous materials. Adsorption collects the target particles by adhesion, while membranes use filtering. I transitioned to membrane research when I became a research associate at Hiroshima University in 1996. I moved to Kobe University when the Graduate School of Science, Technology and Innovation was established in 2016. Today, I also work as a professor in advanced membrane science and technology at this graduate school.
Membranes have various possible applications. Which aspect do you focus on?
Prof. Yoshioka:
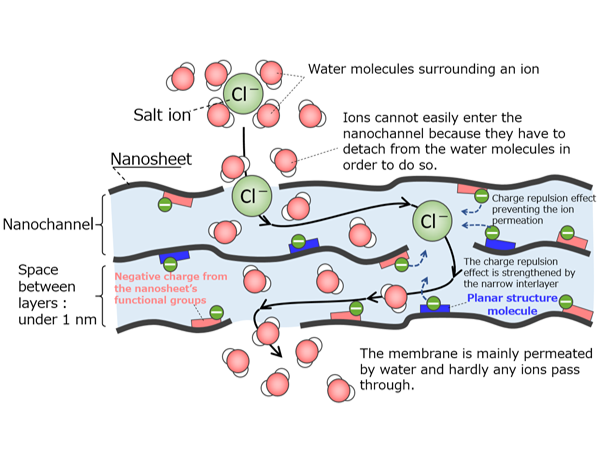
Membranes separate specific substances. Molecules smaller than the diameter of the membrane’s tiny pores can pass through while larger molecules cannot. Organic materials (polymers) are used as membranes for water treatment, whereas inorganic membranes made of ceramic, which I study, are used for high-temperature gas separation. Polymeric membranes made of plastic are cheap and easy to process. However, they lack heat or chemical resistance. In contrast, inorganic membranes made of ceramic are heat and chemical resistant. They can be used to separate substances dissolved in organic solvents such as alcohol, toluene, hexane, or a mixture of organic solvents. Inorganic membranes are also used to separate high-temperature gases at temperatures between 300 °C to 500 °C.
Molecular simulations, which are a cornerstone of my studies on adsorption, calculate the behavior of substances when they pass through membranes at the molecular level. Such simulations improve membrane performance by enhancing structural and material design.
Pursuing the Possibilities of Ceramic Membranes

Could you tell us about the potential of ceramic membranes?
Prof. Yoshioka:
If a petrochemical plant process, which currently separates and refines a particular substance by distilling at various temperatures, can be replaced by a separation process using ceramic membranes, vast amounts of energy could be saved during distillation while simultaneously reducing the amount of CO2 emitted. Additionally, ceramic membranes have the potential to transport hydrogen, which is gaining attention as an environmentally-friendly energy source. Hydrogen is classified as green or blue. Green hydrogen is produced by electrolyzing water using electricity generated from solar or wind energy, whereas blue hydrogen is extracted alongside the CO2 emitted from the hydrocarbons in fossil fuels. For both types of hydrogen, transportation and storage remain a challenge.
As an academic–industrial partnership project, Kobe University is working on research to use ceramic membranes for organic hydrides that can transport and store hydrogen at normal temperature and pressure. This would be a significant advance compared to high-pressure hydrogen gas or liquid hydrogen, whose density increases by applying high pressure or extremely low temperature. When hydrogen and toluene form a chemical bond, it liquefies at room temperature into methylcyclohexane, which has a volume equivalent to several hundred thousandths of hydrogen. Since methylcyclohexane is stable at normal temperatures, it can be used in the existing fuel supply chain, including gas stations. The toluene and hydrogen can be separated at the use site. This is done by heating the methylcyclohexane to 250–300 °C which causes it to vaporize. It is then passed through ceramic membranes to separate the toluene and hydrogen. We plan to reuse the toluene and utilize the extracted hydrogen for fuel cells.
What is your vision for practical applications?
Prof. Yoshioka:
The membrane process for petrochemical plants is still at the lab level. However, we are working with startups focusing on ceramic membranes for hydrogen separation. We are close tobeing able to practically apply our method for transporting and separating hydrogen for fuel cells. We hope to overcome each challenge that we are facing (such as membrane stability) and implement this technology as soon as possible.
The Research Center for Membrane and Film Technology founded the Organization for Membrane and Film Technology Research in 2007 as a general incorporated association. Its mission is to promote academic–industrial partnerships. Today, there are about 80 member companies working on various joint research projects. This includes research that will contribute towards solving global environmental issues, as well as projects directly connected to the companies’ business.
Nurturing Talent
How are you promoting international joint research?
Prof. Yoshioka:
Under the Kobe University Strategic International Collaborative Research Grant program*, we work on joint research with 12 universities in 6 countries and regions such as the United States, Australia, China, and Taiwan. In one project, we are conducting collaborative research with Chung Yuan Christian University in Taiwan, which owns equipment that can measure pores with diameters less than 1 nanometer. The University of Technology Sydney in Australia is investigating how to improve the performance of polyamide, a polymer used in reverse osmosis membranes by mixing it with carbon compounds and graphene. In another project, we collaborate by elucidating the behavior of water using computer simulations. By our leveraging strengths, I believe that Kobe University can advance membrane research together with overseas researchers.
Sending students to overseas universities for international joint research also contributes to cultivating researchers. The Graduate School of Science, Technology and Innovation, which I also work for, has a Doctoral Program for working students. Six graduate students sent by companies have earned or are expected to earn a PhD. in the field of advanced membrane and film technology by the end of the 2022 academic year. Additionally, there are two working students in the first year and the second year of the Doctoral Program, respectively. It is important for us to develop young researchers who will go on to actively participate in research at companies.
Resume
| March 1996 | Withdrew from the Doctoral Program at the Department of Chemical Engineering, Graduate School of Engineering, Kyoto University with the Completion of Course Requirements |
| April 1996 | Became a Research Associate at the School of Engineering, Hiroshima University |
| March 1997 | Completed a PhD (Engineering) at Kyoto University |
| April 2001 | Became Research Associate at the Graduate School of Engineering, Hiroshima University |
| February 2007 | Promoted to Assistant Professor |
| April 2007 | Promoted to Associate Professor |
| April 2016 | Became Professor at the Graduate School of Science, Technology and Innovation, Kobe University |
| April 2019 | Became Professor at the Research Center for Membrane and Film Technology, Kobe University |
*Note
- Strategic International Collaborative Research Grant | Kobe University
Professor Yoshioka’s project has been approved as a Type B project under the Kobe University Strategic International Collaborative Research Grant program.