A collaboration including researchers from Kobe University, University of Tokyo and Tohoku University has successfully identified and disrupted genes in the yeast Pichia pastoris (*1) in order to increase its secretory production of useful proteins (*2). Through a series of processes that involved combining gene disruptions and then serially cultivating (*3) the resulting multiple disruption strains, they developed P. pastoris strains that can produce high yields of useful proteins. It is hoped that this discovery will lead to the development of techniques to improve protein production for biomedical antibodies and industrial enzymes, among other applications.
From Kobe University, the research collaboration included Project Associate Professor ITO Yoichiro and Associate Professor ISHII Jun (both of the Engineering Biology Research Center), and Professor KONDO Akihiko (Graduate School of Science, Technology and Innovation).
These results were published in Communications Biology on June 8, 2022.
Main points
- The researchers used a multi-well formatted high-throughput assay to identify P. pastoris gene disruptions that could increase the secretory production of useful proteins.
- Secretory production of useful proteins could be increased when multiple disruptions to identified genes were combined.
- These genes with multiple disruptions were found to increase secretory production, even when the useful protein or its expression were different.
- By cultivating these multiple-gene disruption strains (i.e. useful protein production strains), the researchers successfully increased secretory production of useful proteins.
Research Background
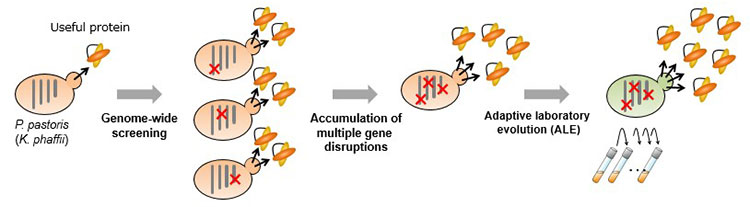
In recent years, attempts have been made worldwide to produce proteins for enzymes (used in industry) and antibodies (used in biomedicine) via secretory production by the yeast P. pastoris (syn. Komagataella phaffii). However, various difficulties hamper this process, for example, P. pastoris has a low production rate for some target proteins. In light of this, the researchers came up with the following approach (Figure 1) aiming towards high productivity of difficult-to-secrete proteins (or proteins with low secretory production) in P. pastoris: 1. Identify effective gene disruptions by screening a library of random genome-disruption strains, 2. Add multiple disruptions to the identified genes, 3. Improve secretory production through serial cultivation.
Research Methodology
Creation of a random genome-disruption library and screening:
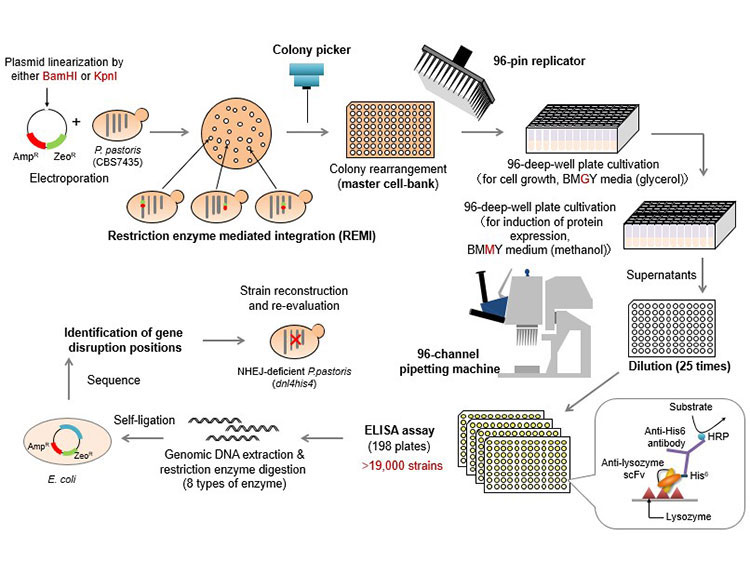
In order to find effective gene disruptions in a random genome-disruption library, the researchers first of all developed a simple method to evaluate how well various genetically modified strains could produce the target proteins (Figure 2). Anti-lysozyme single-chain variable fragment (scFv) antibody (*5) is difficult to secrete in P. pastoris. Using scFv as a model protein, the researchers set up a high-throughput multi-well screening system so that they could easily evaluate the secretion of scFv by a large number of mutant strains. They created a random genome-disruption library from P. pastoris strains that can produce scFv, and using the method explained above, they were able to evaluate the scFv productivity of over 19,000 different strains. From this, they succeeded in identifying six genes in which gene disruption could increase scFv production (five of the six types of gene disruption were new) (Figure 3).
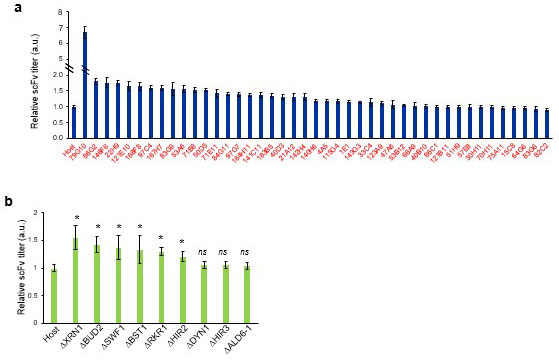
Coincidentally during this screening, they also discovered a mutation in a secretory signal sequence (*6) that greatly increases scFv secretion (79G10 in Figure 3a) (Reference: Ito et al., Avoiding entry into intracellular protein degradation pathways by signal mutations increases protein secretion in Pichia pastoris, Microbial. Biotechnol, 2022.). This is a 1 amino acid mutation (V50A) in the Saccharomyces cerevisiae-derived (*7) MFα signal peptide.
Combining multiple disruptions in the identified useful genes
The researchers proceeded to disrupt the identified genes (obtained through the step above) in a scFv production strain of P. pastoris, revealing that the accumulation of gene disruptions led to increased protein productivity. The strain with multiple disruptions to four of the identified genes demonstrated a 5-fold improvement in productivity compared to the parental strain (Figure 4). In addition, the researchers showed that (multiple) disruptions to these genes had the same effect, not only in the scFv production strain used in the screening, but also in a β-glucosidase (*8) production strain and an scFv strain with a different promoter to the one used in the screening (Figure 4).
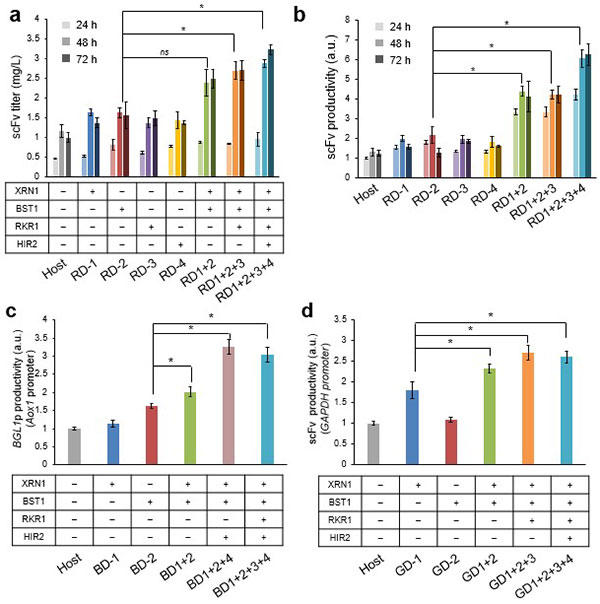
Serial cultivation of strains with (multiple) gene disruption(s)
In the strains with (multiple) disruptions in each gene, they found that the speed of P. pastoris cell multiplication decreased as the gene disruptions accumulated (Figure 4). To restore the multiplication speed in these strains, they repeated the cultivation cycle over 50 times, allowing the yeast to grow in a fresh culture medium each time. This is called Adaptive Laboratory Evolution (ALE). Using this technique, the researchers succeeded in restoring cell multiplication in the majority of the disrupted strains, in addition to boosting their scFv productivity (Figure 5).
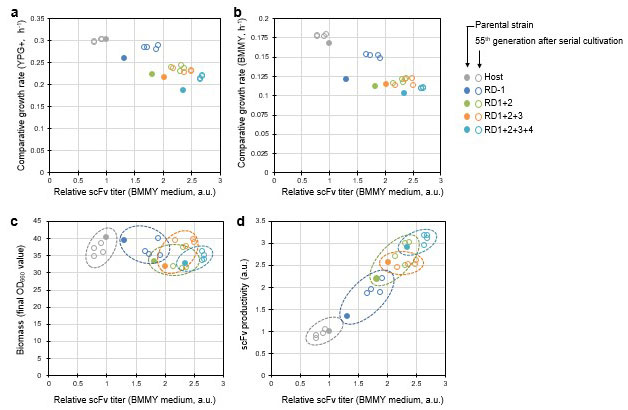
Through the three steps described above (1. Creation of a random genome-disruption library and screening, 2. Combining multiple disruptions in the identified useful genes, 3. Cultivation of strains with (multiple gene disruption(s)), the researchers successfully increased the production of target proteins. This can serve as a method for developing new host strains to increase the production of useful proteins in P. pastoris.
Further Research
Utilizing the insight obtained from this study, the researchers plan to develop P. pastoris stains with even higher productivity of difficult-to-express target proteins.
Glossary
1. Pichia pastoris (Komagataella phaffii)
A species of methylotrophic non-conventional yeast. Its secretory production of proteins is comparatively high when a strong methanol-regulated promoter is used. P. pastoris is not only used as a host for protein production in research but is widely used in industry.
2. Useful protein
This term indicates introduced proteins that have useful applications, including as useful enzymes in industry and as antibodies in biomedicine.
3. Serial cultivation
A method of repeatedly cultivating a bacteria or fungus (in this case, yeast) over and over. After the yeast has been cultivated, a portion is transferred to a fresh medium and the cultivation process begins again.
4. Random genome-disruption library
A cell population with different gene disruptions created by random gene disruptions.
5. Single-chain variable fragment (scFv)
Low molecular weight antibody in which the variable regions of heavy and light chains (VH and VL), the smallest units necessary to recognise antigens, are fused with a flexible peptide linker.
6. Secretory signal sequence
A polypeptide sequence appended upstream of the protein to be secreted.
7. Saccharomyces cerevisiae
A budding yeast and a model organism. S. cerevisiae is the most commonly used yeast with an extensive range of research applications such as in genetics and metabolic engineering. It has been used to make traditionally fermented products since ancient times, including beer, wine, Japanese spirits and bread.
8. β-glucosidase
An enzyme that hydrolyses one molecule of cellulose (disaccharide) into two molecules of glucose.
Acknowledgements
This research received support from the following:
- The Japan Agency for Medical Research and Development (AMED)’s ‘Project Focused on Developing Key Technology for Discovering and Manufacturing Drugs for Next-Generation Treatment and Diagnosis’.
- This research was partly supported by the Ministry of Economy, Trade and Industry (METI)’s ‘Commission for Development of Artificial Gene Synthesis Technology for Creating Innovative Biomaterials’.
- The New Energy and Industrial Technology Development Organization (NEDO)’s project for the Development of Production Techniques for Highly Functional Biomaterials Using Smart Cells of Plants and Other Organisms.
- The JST-Mirai program (JPMJMI17EJ) and the CREST program (JPMJCR21N2) from the Japan Science and Technology Agency (JST).
- A Grant-in-Aid from the Japan Society for the Promotion of Science (JP20K05229).
Journal Information
Title
DOI
10.1038/s42003-022-03475-w
Authors
Yoichiro Ito, Misa Ishigami, Goro Terai, Yasuyuki Nakamura, Noriko Hashiba, Teruyuki Nishi, Hikaru Nakazawa, Tomohisa Hasunuma, Kiyoshi Asai, Mitsuo Umetsu, Jun Ishii* and Akihiko Kondo*
*Corresponding authorJournal
Communications Biology