Immobility or a decline in physical activity is a common cause of muscle loss, but the underlying mechanism of this loss has remained unclear. A research group led by Professor Wataru Ogawa at Kobe University Graduate School of Medicine has recently uncovered how immobilization induces loss of muscle mass.
A reduction in muscle mass impairs the ability to move. It can also lead to various diseases and a consequent shortening of life span. The loss of muscle mass and resultant impairment of physical activity that occurs with age is known as “sarcopenia,” which has become a serious health burden in aging societies. It is well known that exercise increases muscle mass and, conversely, that immobility or a decrease in physical activity reduces it. Moreover, the decline in exercise capacity that accompanies a loss of muscle mass leads to a reduction in daily physical activity and consequent further muscle loss. This vicious cycle is often rapidly accelerated by forced bed rest, such as that associated with surgery or hospitalization for a severe illness. No drug is currently available for the treatment of muscle loss.
Using a newly developed bioimaging technique, Professor Ogawa’s research team made the surprising finding that the concentration of calcium inside muscle cells falls to below normal levels in response to immobilization and that this change initiates muscle loss. The researchers also found that three proteins—Piezo1, KLF15, and IL-6—act sequentially to bring about the loss of muscle mass. Their discovery may provide the basis for development of a drug that can prevent muscle loss. The new findings were published on 15 March 2022 in the online edition of The Journal of Clinical Investigation.
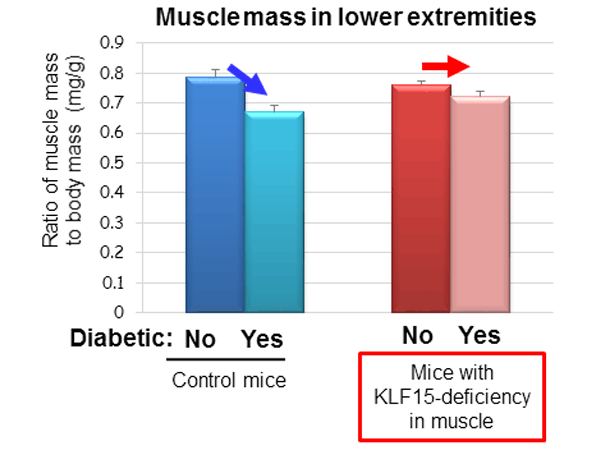
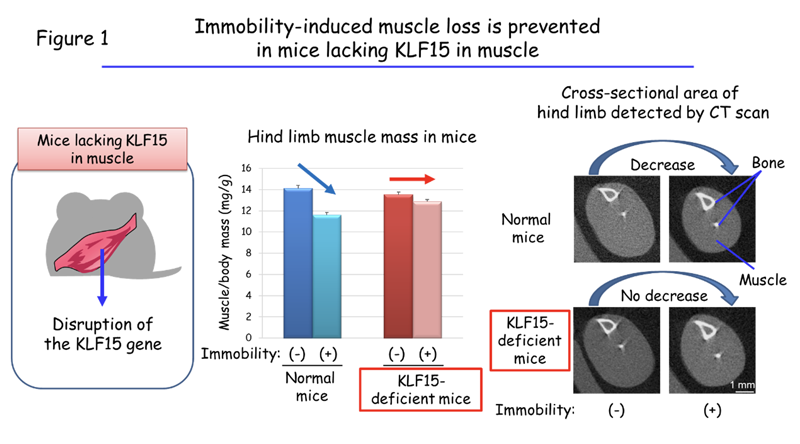
Professor Ogawa’s team showed that limb immobilization caused by cast fixation or the resection of a motor nerve in mice induced muscle loss and an associated increase in the abundance of the protein KLF15 in muscle cells. Furthermore, the immobilization-induced muscle loss was prevented in mice that lack KLF15 specifically in muscle (Figure 1). These results indicate that muscle loss due to immobility is the result of the increased abundance of KLF15.
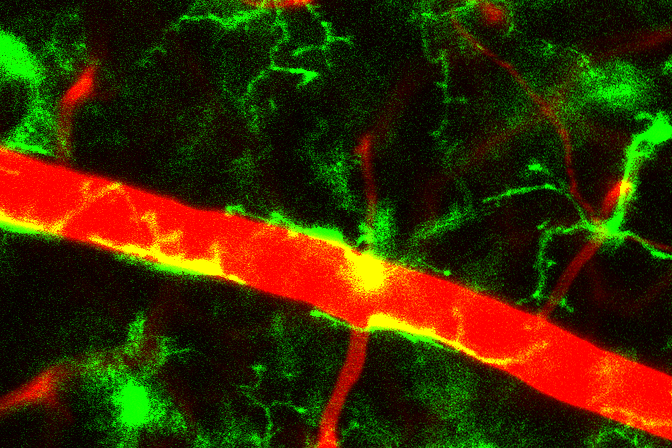
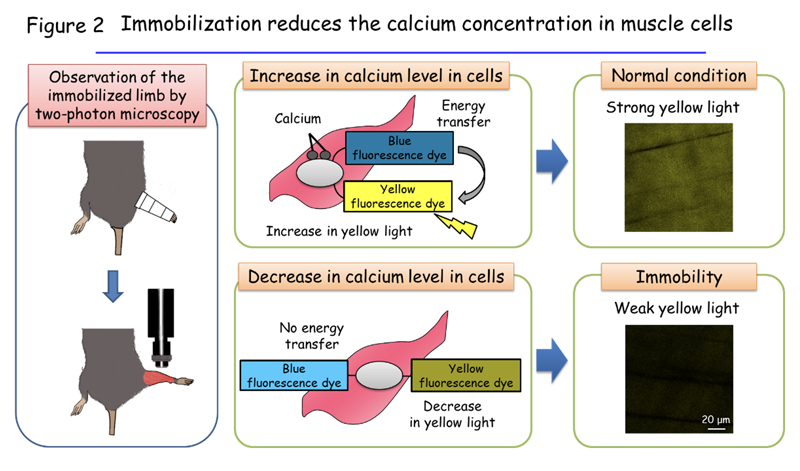
The team next investigated how immobilization increases the amount of KLF15. The researchers found that the decrease in the calcium concentration of muscle cells is responsible for this phenomenon. The concentration of calcium in cells is usually maintained at a low level. However, exposure of cells to certain stimuli induces a rapid increase in the intracellular calcium concentration by a factor of one to two orders of magnitude, and this increase then triggers various reactions within the cells. The research team developed a new bioimaging technique1 that enables very low levels of calcium in muscle cells of living animals to be measured. Using this technique, they found that muscle cells’ low calcium levels under normal conditions decline even further in response to limb immobilization (Figure 2), and that this fall in the basal calcium concentration is responsible for the increase in KLF15 abundance and the decrease in muscle mass induced by immobilization. Various biological phenomena are triggered by a rise in cellular calcium levels, but the induction of a defined biological process by a reduction in the basal calcium concentration has not previously been described.
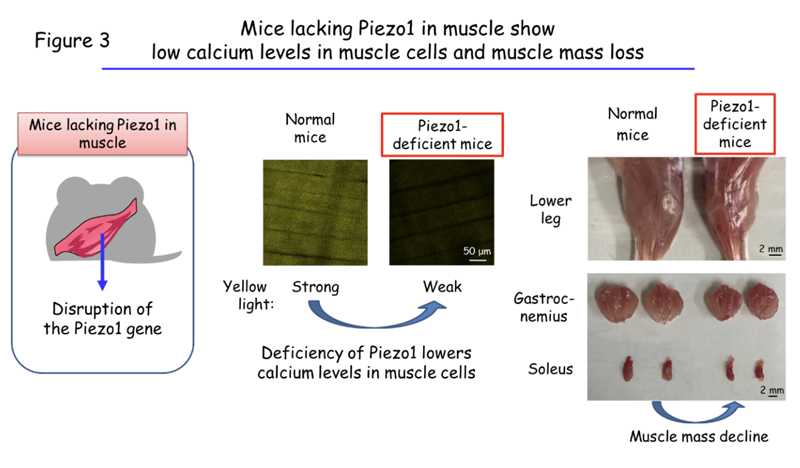
The researchers further investigated how limb immobilization reduces the calcium concentration in muscle cells. They found that expression of the protein Piezo1 in the cells was also reduced by immobilization. Piezo1 serves as a channel that allows the passage of calcium from the external environment into cells in response to mechanical stimulation. Its related protein Piezo2 contributes to the generation of a sense of touch.2 Professor Ogawa’s team also showed that mice that lack Piezo1 specifically in the muscle had reduced calcium levels in muscle cells and manifested muscle loss (Figure 3).
In addition, the team showed that the protein IL-6 (interleukin-6)3 plays an important role in immobilization-induced muscle atrophy. IL-6 was found to be secreted from muscle cells in response to loss of Piezo1 and the increase in KLF15 abundance, and treatment of mice with antibodies that block the action of IL-6 prevented the immobilization-induced loss of muscle mass.
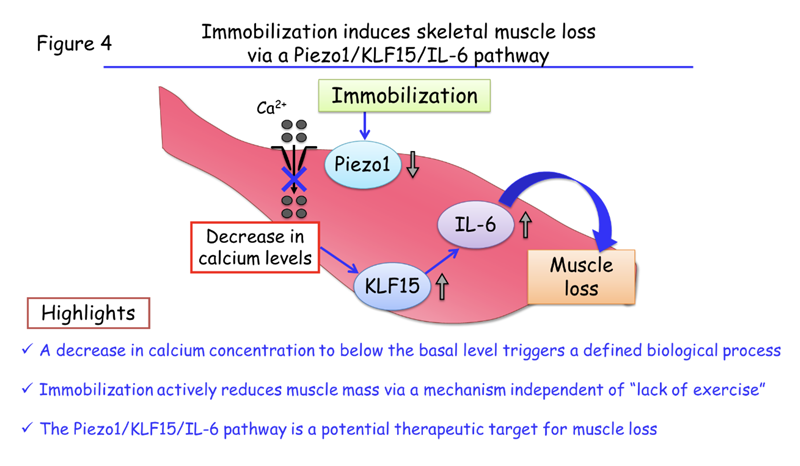
This new study has thus revealed that a decline in the calcium concentration of muscle cells to below the basal level, which is caused by a reduction in expression of the Piezo1 channel, triggers immobilization-induced muscle loss through the action of KLF15 and IL-6 (Figure 4). The research team validated this role of the Piezo1–KLF15–IL-6 pathway in immobility-induced muscle loss using tissue samples from patients with immobilization-induced muscle atrophy. Neither a drop in intracellular calcium levels nor the Piezo1–KLF15–IL-6 pathway has previously been implicated in such muscle loss.
The immobility-induced loss of muscle mass has previously been proposed to be the result of a lack of stimuli for muscle growth normally provided by exercise. The current study, however, shows that immobility itself turns on a signaling pathway that actively reduces muscle mass and is independent of exercise-induced muscle growth.
Given the current lack of an effective medicine for treatment of muscle loss, the new finding that antibodies responding to IL-6 prevented immobilization-induced muscle atrophy in mice is promising. However, there is a concern that administration of such antibodies to patients might have side effects on the immune system. Professor Ogawa comments that “If we can develop a drug that strengthens the function of Piezo1 or weakens the function of KLF15, it could lead to a breakthrough in the treatment or prevention of muscle loss.” Professor Ogawa’s group has already started a project with the aim of developing such drugs with the support of the Japan Agency for Medical Research and Development.4
Highlights
- A decrease in calcium concentration to below the basal level triggers a defined biological process.
- Immobilization actively reduces muscle mass via a mechanism independent of “lack of exercise”.
- The Piezo1/KLF15/IL-6 pathway is a potential therapeutic target for muscle loss
Glossary
- 1 Bioimaging technique
- A technology that allows visualization of various reactions in organs or cells of living animals. The research group used two-photon microscopy to observe the inside of muscle cells. For the first time in the world, they detected a change in the intracellular calcium level in skeletal muscle of mice. This analysis was performed in a collaboration with Professor Hiroaki Wake of Nagoya University and Professor Takahiro Adachi of Tokyo Medical and Dental University.
- 2 The generation of a sense of touch
- Piezo1 opens in response to mechanical deformation of the cell membrane and thereby allows the transfer of calcium from outside to inside of the cell. Its related protein Piezo2 is expressed in Merkel cells and is responsible for generating a sense of touch on the skin. When we touch something, cellular deformation triggered by the touch results in the opening of Piezo2 and generates a neural signal for touch. The 2021 Nobel Prize in Physiology or Medicine was awarded to Dr. Ardem Patapoutian for this discovery of the role of Piezo2 in the sense of touch.
- 3 IL-6
- A cytokine that plays an important role in the regulation of inflammation in various settings. Antibodies to IL-6 are used to treat rheumatoid arthritis or severe pneumonia caused by COVID-19.
- 4 Japan Agency for Medical Research and Development (AMED)
- A national agency established to promote and support the research and development of pharmaceuticals, medical devices, and medical technology.
Journal Information
- Title
- “A Piezo1/KLF15/IL-6 axis mediates immobilization-induced muscle atrophy”
- DOI
- 10.1172/JCI154611
- Authors
- Yu Hirata, Kazuhiro Nomura, Daisuke Kato, Yoshihisa Tachibana, Takahiro Niikura, Kana Uchiyama, Tetsuya Hosooka, Tomoaki Fukui, Keisuke Oe, Ryosuke Kuroda, Yuji Hara, Takahiro Adachi, Koji Shibasaki, Hiroaki Wake, Wataru Ogawa*
*Corresponding author - Journal
- Journal of Clinical Investigation